A Star Surgeon Left a Trail of Dead Patients—and His Whistleblowers Were Punished

Dr. Paolo Macchiarini, then a professor of Regenerative Medicine at Karolinska Institute in Stockholm (Sweden), looks on during a plenary session at the Science of the Future international scientific conference, on September 17, 2014.
[Editor's Note: This is the first comprehensive account of the whistleblowers' side of a scandal that rocked the most hallowed halls in science – the same establishment that just last week awarded the Nobel Prize in Medicine. This still-unfolding saga is a cautionary tale about corruption, hype, and power that raises profound questions about how to uphold integrity in scientific research.]
When the world-famous Karolinska Institutet (KI) in Stockholm hired Dr. Paolo Macchiarini, he was considered a star surgeon and groundbreaking stem cell researcher. Handsome, charming and charismatic, Macchiarini was known as a trailblazer in a field that holds hope for curing a vast array of diseases.
It appeared that Macchiarini's miracle cure was working just as expected.
He claimed that he was regenerating human windpipes by seeding plastic scaffolds with stem cells from the patient's own bone marrow—a holy grail in medicine because the body will not reject its own cells. For patients who had trouble breathing due to advanced illness, a trachea made of their own cells would be a game-changer. Supposedly, the bone marrow cells repopulated the synthetic scaffolds with functioning, mucus-secreting epithelial cells, creating a new trachea that would become integrated into the patient's respiratory system as a living, breathing part. Macchiarini said as much in a dazzling presentation to his new colleagues at Karolinska, which is home to the Nobel Assembly – the body that has awarded the Nobel Prizes in Physiology or Medicine since 1901.
Karl-Henrik Grinnemo was a young cardiothoracic surgeon and researcher at Karolinska in 2010, when Macchiarini was hired. "He gave a fantastic presentation with lots of animation and everyone was impressed," Grinnemo says of his first encounter with Macchiarini. Grinnemo's own work focused on heart and aortic valve regeneration, also in the field of stem cell research. He and his colleagues were to help establish an interdisciplinary umbrella organization, under Macchiarini's leadership, called the Advanced Center for Translational Regenerative Medicine, which would aim to deliver cures from Karolinska's world-class laboratories to the bedsides of patients in desperate need.
Little did Grinnemo know that when KI hired Macchiarini, they had ignored a warning that the star surgeon had been accused of scientific misconduct by a colleague who had worked with him at the University of Florence. That blind eye would eventually cost three patients their lives in Sweden.
"A MIRACLE CURE"?
It has been said that if all you have is a hammer, everything looks like a nail, and it wasn't long before Macchiarini announced that he had a patient in dire need of one of the new artificial tracheas. The patient, a native of Eritrea who had emigrated to Iceland, had a slowly growing tumor on his trachea. Macchiarini had previously generated new windpipes from human donor tracheas outside of Sweden, but the Icelandic patient was the first to receive a synthetic trachea implant at Karolinska University Hospital. Macchiarini had already performed a similar procedure with decellularized donor tracheas on other patients around Europe, but not much was known at the time about their outcomes.
Of course, to justify a radical procedure such as removing a patient's trachea, one would need compelling evidence of effectiveness in animal studies, as well as an exhaustion of all other treatment alternatives. Macchiarini claimed that both conditions were met. He performed the implantation of the synthetic trachea as if he had received a hospital exemption. This is comparable to what the U.S. Food and Drug Administration classifies as "compassionate use," a procedure performed only in extreme circumstances, usually when the patient is terminal, and when no available alternative has worked.
Macchiarini personally invited Grinnemo to watch the all-day surgery, and, once the transplant was done after 10 grueling hours, Macchiarini asked him to close the patient. Then the 36-year-old man was transferred to another hospital, where Grinnemo and other attending physicians had little opportunity to follow his long-term recovery.
Two months later, Macchiarini approached Grinnemo with an invitation to be one of multiple co-authors on a paper about the case targeted for the New England Journal of Medicine. This was a huge opportunity for a junior researcher, and Grinnemo gladly agreed to write a one-month follow-up report on the Icelandic patient's clinical condition. He consulted the patient's medical records, which described a man with an infection in one lung but otherwise doing well, and wrote up his contribution. The patient had already been transferred back to Iceland by then and was home from the hospital. It appeared that Macchiarini's miracle cure was working just as expected.
But the ground was beginning to shake.
"We cannot find one word of evidence that points to regeneration induced by stem cells."
On September 2, 2011, three months after the Icelandic patient's surgery, a professor in Leuven, Belgium sent a written warning to KI's vice chancellor, Harriett Wallberg-Henriksson, stating that Macchiarini was guilty of prior research misconduct. This letter was forwarded to the new president at KI, professor Anders Hamsten, urging him to put a halt to more synthetic trachea implants. The accusations were grave.
Professor Pierre Delaere at Kathiolieke Universiteit asserted that synthetic tracheas coated with bone marrow cells did not, as Macchiarini had claimed, transform into living tracheas. He cited "countless" failures in animal experiments and called the outcome of Macchiarini's previous human surgeries "disastrous…half the patients died. The others are in a palliative setting….We cannot find one word of evidence that points to regeneration induced by stem cells."
Once again, KI simply ignored the warning, and Grinnemo and the 24 co-authors on the splashy academic paper about the latest surgery didn't even know about it. In the meantime, the New England Journal of Medicine rejected it for lacking a longer follow-up on the patients and missing data on how well the implants had integrated with the patient's respiratory system, so Macchiarini submitted it to The Lancet instead.
And he kept performing his experimental surgeries.
Soon there was a second transplant patient, a 30-year-old American man named Christopher Lyles. After his operation at KI, he returned to the U.S and the Swedish doctors were unable to follow his progress. Three months after his surgery, they learned that he had died at his home.
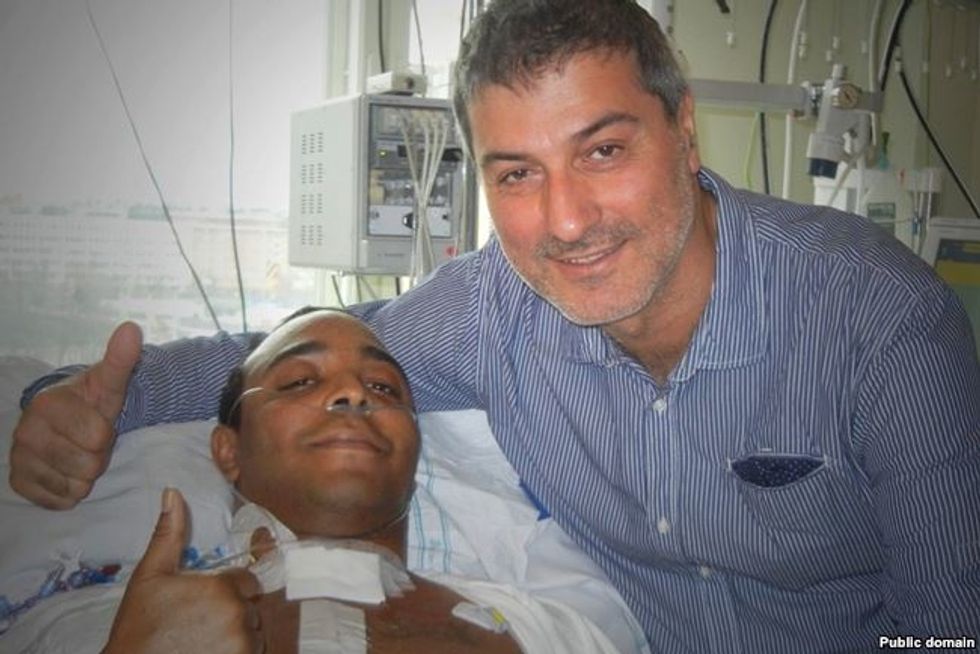
Paolo Macchiarini with Christopher Lyles, the American patient on whom he performed a trachea transplant in Stockholm in 2011. Lyles died a few months later.
Only four months after Lyles died, the third patient, a 22-year-old Turkish woman, received one of Macchiarini's grafts. In all three patients, Macchiarini had claimed that they were in dire straights—terminal if not for the hope of a trachea transplant, and he claimed a hospital exemption in all three cases. In fact, Grinnemo says, all three had been in stable condition before their surgeries—a reality Macchiarini did not share with his collaborators and co-authors on two academic papers about the surgeries that were subsequently published in The Lancet.
The Turkish woman's story is especially tragic. The young woman had initially undergone surgery elsewhere to fix an unrelated problem—hand sweating--but wound up with an accidentally damaged trachea that set her on a course of utter devastation. She sought help from Macchiarini, but his graft operation left her "living in hell," says Grinnemo. In intensive care afterward, her airways were producing so much mucus that they had to be cleared every four hours around the clock. The procedure "is like someone keeping your head under water every fourth hour until you almost suffocate to death. This is something that you wouldn't wish on your worst enemy," says Grinnemo.
By the spring of 2013, six months after Macchiarini's operation, the graft began to collapse. Several metal stents were inserted into her airways, but each one only worked for a short while. Macchiarini decided to remove the first plastic trachea and implant a new one. It seemed she couldn't get any worse, but after the second transplant, the young woman further deteriorated. Her airway secretions only increased; she had to undergo thousands of bronchoscopies, where an instrument was pushed down her throat into her lungs, and hundreds of surgeries during her three-year stint in the intensive care unit. Her body couldn't tolerate much more.
The whistleblowers realized that, despite Macchiarini's claims of successful operations in several now-published papers, the patients had been mutilated.
Grinnemo, together with fellow KI physicians Matthias Corbascio, Oscar Simonson and Thomas Fux, who were all involved in the care of the Turkish woman, became alarmed when the Icelandic patient came back to their hospital in the fall of 2013 with similar complaints. They realized that, despite Macchiarini's claims of successful operations in several now-published papers, the patients had been mutilated.
Both the Icelandic patient and the Turkish woman were too incapacitated to speak for themselves, so in the late fall of 2013, Grinnemo and his three concerned colleagues reached out to the patients' relatives seeking permission to review their medical records. It took weeks to receive the permissions, but once they did, what they found stunned them.
The Icelandic patient had developed fistulas (holes) between the artificial trachea and his esophagus, and had been fitted with several stents. Soon his esophagus also had to be removed, which Macchiarini was aware of. He should have reported these complications in the articles on which he was lead author, Grinnemo contends, and also should have informed his co-authors, each of whom had been responsible for writing up discrete sections of the papers. But Macchiarini had described each transplant as a success and had greatly exaggerated, if not outright lied, about how each patient had fared.
THE WHISTLEBLOWERS FIGHT BACK
Grinnemo and several other suspicious colleagues decided to launch an investigation. The result was a 500-page report identifying the synthetic tracheas as the problem and revealing that Macchiarini had falsified data and suppressed critical information in his reporting. He had even invented biopsies of the grafts, claiming that the marrow cells had populated them with functioning epithelial cells, while there was no real evidence of the patients' cells growing to line the tracheas.
The whistleblowers also discovered that Macchiarini had never received ethical clearance from Sweden's Human Ethical Review Board, nor had he gotten approval for his plastic tracheas from the Medical Product Agency, the Swedish counterpart to the FDA. He had relied entirely on his ability to do the surgeries under the hospital exemption, which he made everyone believe that he had obtained thanks to his star power.
What Macchiarini was doing, the investigators realized, was experimentation on living human subjects; he had circumvented the normal oversight protocols that exist to protect such subjects.
At a procedural meeting with his colleagues, including Dr. Ulf Lockowandt, the head of Karolinska University Hospital's Department of Cardiothoracic Surgery, Macchiarini dismissed the patients' complications as "manageable."
But among the large interdisciplinary team whose members had knowledge only of their own discrete specialties, doubts about Macchiarini's technique were festering. Complications in the patients only worsened when the tracheas inevitably began to collapse. There was a bursting open of sutures, holes in tissues adjacent to the implants, the disintegration of tissues that clogged bronchial passages. In far more than half of all the patients Macchiarini had operated on in several countries, patients died a lingering and agonizing death.
The last thing the whistleblowers expected was for the full weight of the institution to come crashing down against them.
When Grinnemo and his fellow investigators dug all this up, they decided they had to report it to the very top of Karolinska, to the institute's president, Anders Hamsten, so that he could stop Macchiarini from performing any further transplants. The last thing the whistleblowers expected was for the full weight of the institution to come crashing down against them.
"THEY WANTED TO SILENCE EVERYTHING"
KI had ample reason to sweep criticisms of Macchiarini under the rug. Up to 100 patients were about to be recruited for an international clinical study in which Macchiarini would do his implants—a nightmarish prospect considering his track record. But KI stood to receive millions of dollars in a government grant to conduct the study across Europe and Russia.
Still other incentives existed for KI to suppress Macchiarini's record. Plans were underway to establish a stem cell center in Hong Kong with over $45 million provided by a wealthy Chinese businessman. At the center, Macchiarini would be able to do his trachea transplants on patients in Asia. And in addition to the financial incentives to keep Macchiarini's brand associated with KI, many high-powered individuals were involved in his initial recruitment and didn't want their reputations tarnished, Grinnemo says. KI not only ignored the whistleblowers' allegations; punishment against them was swift and decisive.
On March 7, 2014, Grinnemo and the other whistleblowers met with Dr. Hamsten, in addition to two of Macchiarini's supervisors and the director of KI's Regenerative Network. They presented their findings and requested an official investigation by KI, including scrutiny of the now-six published research papers in which Macchiarini had claimed the success of his implants in humans. The whistleblowers also told the leadership about some rat studies Macchiarini had published in a prestigious journal that appeared to rely on falsified data.
Instead of the welcoming reception they expected, the room bristled with hostility. "I basically forced them to agree to an investigation," Grinnemo says, "but it was a very tough meeting. The feeling I got was that they wanted to silence everything and that they would continue to silence me and the other whistleblowers. We were already feeling the backlash."

From the left, whistleblowers Matthias Corbascio, Oscar Simonson, Thomas Fux and Karl-Henrik Grinnemo.
Previously, Grinnemo had confronted Macchiarini with questions about patients he had implanted in Russia prior to his stint at Karolinska. "Paolo Macchiarini realized we were onto something and he became very angry. He said he would do everything in his power to make my life miserable," Grinnemo recalls.
Macchiarini made good on his threat by filing a complaint about Grinnemo with the Swedish Research Council, the main funder of research in Sweden. At the time, Macchiarini and Grinnemo had jointly submitted a grant application on an aortic valve regeneration project, which the Council had approved. Macchiarini suddenly complained that Grinnemo had stolen his data on aortic valve regeneration, even though, unlike Grinnemo, Macchiarini was not a heart surgeon and had conducted no research on heart structures. In reality, all of the data had been generated by Grinnemo. The Council did a review and concluded that Grinnemo had not stolen the data, but Macchiarini spread rumors throughout KI that the young researcher was guilty of scientific misconduct. "He wanted to discredit me because he knew I was dangerous and he wanted to stop anyone from believing me," Grinnemo says.
In spite of the findings from the Council that he had committed no scientific misconduct, KI opened an investigation—not of Macchiarini, but of Grinnemo himself. It soon became clear that KI also wanted to discredit Grinnemo and to silence any possible rumors about Macchiarini's conduct. The whistleblowers continued to push forward, however, and over a period of several weeks they wrote to president Hamsten four times, asking that KI investigate the deadly transplants still being promoted by Macchiarini as some kind of miracle cure.
After four written requests, Hamsten replied that if the whistleblowers had concerns about Macchiarini, they should contact their supervisors or write a formal complaint. But the whistleblowers had already contacted several individuals in supervisory roles who had made it clear that they wanted nothing to do with the affair. It was obvious that KI would resist any investigation of Macchiarini and that no one, outside of the whistleblowers, wanted to take any responsibility for what could amount to a major scandal at one of the world's most powerful academic institutions.
The whistleblowers had another hostile and unproductive meeting with several doctors at KI with whom they shared a letter they had written to the journal Nature Communications, which published Macchiarini's article on rat experimentation, urging them to investigate whether he had falsified the data. Once again, the whistleblowers met with a wall of resistance. Grinnemo was now discredited because of the aortic valve grant application, the doctors reminded him, and no investigation or retraction of the Nature Communications article would be pursued.
In June 2014, KI made its retaliation against Grinnemo official by putting its legal counsel in charge of its investigation of his grant application. The university's ethical board then concluded that Grinnemo should have informed Macchiarini more clearly that he submitted the application to the Swedish Research Council and that he should have obtained a written acceptance from Macchiarini before proceeding with the application. KI could not find Grinnemo guilty of research misconduct, but accused him of "carelessness" regarding the usage of data—which was his own data all along.
A few years later, Grinnemo was totally cleared by both the Central Ethical Review Board and KI. However, the rumors surrounding the investigation and the finding that he hadn't "used data correctly" in a grant application had done their damage to his reputation. Since then, he has not received a single research grant. "You can't appeal the findings," Grinnemo says. "I don't know if I will ever get more research money. I'm totally dead."
The whistleblowers made multiple appeals to Dr. Lockowandt, the head of the Department of Cardiothoracic surgery, for an investigation into Macchiarini's implants, but they were stonewalled from the beginning. Lockowandt did nothing.
"The heads of departments at the KUH and KI didn't actually have that much power," Grinnemo explains. "Dr. Lockowandt thought he was fighting for his own career and position. He's basically a good person who decided to go the route of an administrator, and if you have conflicts with your superiors, your career will be over." In other words, a real investigation of Macchiarini's record could not happen with so much money and prestige riding on the continued presence of the star surgeon.
By August 11, 2014, the whistleblowers had made repeated requests of Dr. Hamsten for a meeting to present the data inconsistencies between Macchiarini's patients' medical records and what he had reported in numerous articles, all published in prestigious medical journals. When they finally received the answer—a cold instruction to submit a written notification to the heads of their departments—it was clear that KI was giving them the runaround.
But rather than simply ignore the whistleblowers, KI apparently decided to double down, trying to discredit them in an intimidation campaign.
KI even went so far as to force the chief medical officer of Karolinska University Hospital, Johan Bratt, to report the whistleblowers to Swedish police, claiming that they violated the law and the patients' privacy when they went through the patients´ charts and submitted their appeals for investigation to KI and the Central Ethical Review Board. KI claimed that their report revealed the identities of patients, even though they had been careful to anonymize all the information. The police interrogated several of the whistleblowers and concluded that they had done no wrong, but the incident made it clear how low KI would sink in its desire to harass them.
"You can't appeal the findings. I don't know if I will ever get more research money. I'm totally dead."
In private, Grinnemo's colleagues supported him, but feared coming forward out of the fear of losing their jobs. Grinnemo himself was in a tough spot. "I knew it would be difficult for me to do research but I hoped my position as a surgeon was secure," he says. "But after the New York Times article, I realized even that position was not as safe as I had thought."
THE MEDIA CATCHES ON -- WITH A PRICE
On November 24, 2014, The New York Times published a front-page story about Paolo Macchiarini based on the whistleblowers' investigation, which had leaked to the press. Officials at KI suspected one or more of the whistleblowers of being the leakers, but the publicity forced the top brass to at least appear to act. The next day they asked Dr. Bengt Gerdin, a professor of surgery at Sweden's Uppsala University, to do an investigation of Dr. Macchiarini. It's hard not to conclude that, after months of stonewalling on an institutional investigation, the Times article compelled them to do something. But KI still did not take any of the pressure off of Grinnemo and his three fellow whistleblowers.
One by one, each was informed that he would receive a formal warning from Dr. Lockowandt, the head of the cardiothoracic clinic, alleging that they had violated patient privacy by reading medical records. The whistleblowers countered that they had informed consents. They also asked for a meeting with Lockowandt and KI's attorneys, to which they brought a union representative and someone from the Swedish version of the American Medical Association. The union representative informed KI's attorneys that the doctors were actually required by law to consult a patient's medical records when the patient's life is in danger. Not doing so would have been a crime. Karolinska backed off on the formal warnings (which would have been the last step before actual termination) after that. But they found other ways to retaliate.
One whistleblower, Oscar Simonson, had been offered a residency at Karolinska University Hospital, but that offer was withdrawn without explanation. Grinnemo had expected to receive an advisor position in cardiothoracic surgery, but that promotion also evaporated. In addition, the number of surgeries he was tapped to perform was reduced and he was relegated to doing the "less popular" standard heart surgeries that began late in the afternoon and evenings.
The grinding day-to-day pressure on the whistleblowers never let up. On December 19, 2014, Dr. Lockowandt informed all four that they had been on the verge of being fired, but that hospital attorneys changed their minds at the last minute. By then not only were their reputations in tatters, but they had invested an estimated 10,000 hours of labor investigating Macchiarini's misconduct, appealing to KI, and defending themselves against KI's harassment.
When interviewed for this article, Grinnemo said, "I have never had a single day of vacation from this situation. In addition to dealing with it, I've been doing surgery and taking care of patients. I've had trouble sleeping, and it has affected my family. I haven't been able to focus on my family, and I feel guilty toward my kids." Of all the whistleblowers, Grinnemo seems to have received the brunt of the backlash.
KI was finally pushed to further action by yet more negative coverage of the Macchiarini affair in the media. In January 2015, Swedish National Television aired an exposé covering the Macchiarini surgeries and the desperate plight of the patients. In response, the Swedish public demanded that KI make a course correction. On February 19, KI withdrew all of its threats of formal warnings to the whistleblowers.
As the press event began, KI called the heads of the whistleblowers' departments to tell them to make sure the four didn't attend.
However, progress was incremental. On April 16, KI's ethical committee, which had done its own investigation, acquitted Macchiarini of allegations of scientific misconduct. This is the same university ethical board that had reprimanded Grinnemo over his usage of data in the aortic valve grant application.
The whistleblowers maintain that throughout the summer of 2015, KI was still far more focused on covering up the Macchiarini affair than on getting to the bottom of it. On May 13, the professor from Uppsala submitted the results of his independent investigation, in which he concluded that seven out of seven published articles in which Macchiarini was the lead author entailed the fabrication of data.
KI ignored the report. In August 2015, KI's president announced that Macchiarini had been cleared of all charges of scientific misconduct and that, magically, ethical approvals existed for the patient from Iceland. Macchiarini got a reprimand for being "a little sloppy" in his published descriptions of his patients. Then KI, eager to placate the public and salvage its reputation, held a press conference to announce the presumed innocence of its star surgeon.
As the press event began, KI called the heads of the whistleblowers' departments to tell them to make sure the four didn't attend, according to Grinnemo.
"They seemed to think we would come crashing in to the press conference and make a scene. It's ridiculous, but that's what they thought," says Grinnemo.
Around this time, KI asked that the whistleblowers compile and forward all of their correspondence with the independent investigator on the grounds that they were suspected of manipulating his investigation. The accusation went nowhere; the whistleblowers had barely spoken with him.
Then came a request from KI's IT department for the whistleblowers to compile and submit all of their emails for the preceding year. They were simply told that "an anonymous person" had made the request.
Throughout 2015, KI continued to go after the whistleblowers aggressively. That August, they were so discouraged that they felt they would never obtain any additional grants from the Swedish Research Council or any other funding organizations, and that their academic careers were over. To add insult to injury, a Swedish newspaper published an article defending Macchiarini and concluding that he was not guilty of violating the Helsinki Declaration, a statute put into effect after World War II protecting all humans from unauthorized medical experimentation.
THE TIDE TURNS, BUT REDEMPTION IS ELUSIVE
Then in November, they received a request from a Swedish filmmaker to be interviewed about the Macchiarini affair. Not knowing what angle the film was expected to take, they each put in hours in front of the camera. They wouldn't know the results of their interviews until January 2016, when the three-part documentary, "The Experiments," aired on Swedish television. The film documented the tortuous death of a Russian woman and the suffering of other patients who had received Macchiarini's implants.
That same month, a devastating article on Paolo Macchiarini was published in the American magazine Vanity Fair. Titled "The Celebrity Surgeon Who Used Love, Money and the Pope to Scam an NBC News Producer," the article revealed Macchiarini as an even more prolific fabulist and liar than anyone had remotely suspected. Not only did he fabricate data for multiple scientific papers, he had also lied about everything from his alleged medical training and celebrity connections to his personal relationship status.
Ironically, the woman who ultimately dismantled Macchiarini was Benita Alexander, a former producer for NBC News who was at one point engaged to marry him in a lavish ceremony that Macchiarini promised would be officiated by Pope Francis. Except that he didn't know the Pope, and he was already married to one woman and living with another.
Her story of heartbreak infuriated the public. The full list of people who had believed Macchiarini's almost countless fabrications may never be known—a tribute to his considerable personal charisma. But after the "The Experiments" and the Vanity Fair article, the public had had enough of Paolo Macchiarini. They demanded that KI's president step down and that Macchiarini be fired.

TV producer Benita Alexander appeared as a guest on Dr. Oz's show on February 14th, 2018 to discuss Dr. Macchiarini's deception. "He railroaded my life," she said.
In February 2016, there was a cascade of resignations and firings at KI. First, president Anders Hamsten stepped down. Then several top KI officials, including the General Secretary of the Nobel Assembly, the Dean of Research, and an advisor to KI's president, were either fired or stepped down. On March 3, several members of the board were replaced. The whistleblowers received an award for coming forward by an organization called Transparency International, but instead of heaving a sigh of relief, they only felt a continued sense of foreboding.
"We all felt very vulnerable because we knew that KI would retaliate in some way," says Grinnemo. A fellow whistleblower, Dr. Corbascio, gave an interview on a prime time news program saying that KI was a corrupt institution and should apologize to the patients' families and even pay them for their suffering. After that, both he and another colleague came under intensified scrutiny at work. They say that their supervisors, who were deeply involved in collaborations with Macchiarini, watched everything they did, apparently looking for a reason to fire them.
Grinnemo and Simonson both left KI to work for Uppsala University. But the lasting effects of the scandal followed them there. They still couldn't obtain any grants for new research, and other scientists at KI and elsewhere were unwilling to collaborate with them for fear of their own work being "tainted" by association.
On March 23, 2016, Paolo Macchiarini was finally sacked by KI. Still, the whistleblowers couldn't claim victory.
"Our aim," says Grinnemo, "was not to get him sacked but to stop the grafts, and we knew he would continue to do them in other countries. The clinical trial aiming to recruit 100 or so patients hadn't been halted. We tried to warn the Russian authorities and the EU grant office, and wanted them to stop the grant to Macchiarini. There was no response, so at that time we didn't know if the clinical trial would go forward."
Still, there was reason to hope. News of Macchiarini's scientific fraud, not to mention his personal debacle with Benita Alexander, had made its way around scientific circles in Germany and Britain, where a new investigation began.
Eventually, the entire board at Karolinska was replaced. Under its new president, the institute issued a decree this past summer finding the now thoroughly disgraced Macchiarini guilty of scientific misconduct, and concluding that six of his research papers should be retracted.
But in a cruelly ironic twist, KI took the whistleblowers' own investigation and turned it against them. KI's report found Grinnemo also guilty of scientific misconduct for apparently falling short in the care of the Icelandic patient, even though his role in the case had been minimal. It was like a punch in the gut, because the judgment cast Grinnemo as equally blameworthy to Macchiarini. It also failed to recognize that he had long ago not only withdrawn his name from the offending paper, but lobbied for years to have it retracted.
"This sends the message that whistleblowers in research will be punished. That's a serious problem."
The KI report also established the new category of "blameworthy" to describe two of the whistleblowers for their roles as co-authors in some of the papers. The whistleblowers did not receive a chance to respond to the new accusations before a decision was made to publicly reprimand them.
That decision can't be appealed.
Simonson told Science Magazine, "This sends the message that whistleblowers in research will be punished. That's a serious problem."
These days, Macchiarini is lying low but still publishing his supposed stem cell research, most recently on baboons. A paper published in March of this year in the Journal of Biomedical Materials lists his affiliation as Kazan Federal University in Russia, but in April 2017, the university fired him. He's rumored to be living in Italy and couldn't be reached for this article. He was investigated for criminal activity in Sweden and the case was closed without charges, but Grinnemo says that another prosecutor is now considering whether to bring charges against him for "aggravated manslaughter."
At KI, only Karin Dahlman Wright, who was the Institute's acting president during several months of these events, responded to a request for comment, but she claimed a near-total unawareness of the whistleblowers' narrative. Other officials there declined to be interviewed.
KI's clinical trial that was aiming to recruit new patients for biologically engineered tracheas is no longer happening. The European Commission posted on their research portal that the trial ended on March 31, 2017, stating: "Grant Agreement terminated."
As for Grinnemo, Simonson, Corbascio and Fux, they are still fighting for their careers. Grinnemo is currently suing KI for a chance to defend himself against its accusations of scientific misconduct. He's also claiming damages for lost grant funding, thousands of hours spent defending himself, and harm to his reputation. Whether he will prevail in court remains to be seen.
"KI did a very good job of destroying our careers," says Simonson. "They didn't do anything else well, but they did a very thorough job of that."
New implants let paraplegics surf the web and play computer games
Rodney Gorham, an Australian living with ALS, has reconnected with the world, thanks to a brain-machine interface called the Stentrode.
When I greeted Rodney Gorham, age 63, in an online chat session, he replied within seconds: “My pleasure.”
“Are you moving parts of your body as you type?” I asked.
This time, his response came about five minutes later: “I position the cursor with the eye tracking and select the same with moving my ankles.” Gorham, a former sales representative from Melbourne, Australia, living with amyotrophic lateral sclerosis, or ALS, a rare form of Lou Gehrig’s disease that impairs the brain’s nerve cells and the spinal cord, limiting the ability to move. ALS essentially “locks” a person inside their own body. Gorham is conversing with me by typing with his mind only–no fingers in between his brain and his computer.
The brain-computer interface enabling this feat is called the Stentrode. It's the brainchild of Synchron, a company backed by Amazon’s Jeff Bezos and Microsoft cofounder Bill Gates. After Gorham’s neurologist recommended that he try it, he became one of the first volunteers to have an 8mm stent, laced with small electrodes, implanted into his jugular vein and guided by a surgeon into a blood vessel near the part of his brain that controls movement.
After arriving at their destination, these tiny sensors can detect neural activity. They relay these messages through a small receiver implanted under the skin to a computer, which then translates the information into words. This minimally invasive surgery takes a day and is painless, according to Gorham. Recovery time is typically short, about two days.
When a paralyzed patient thinks about trying to move their arms or legs, the motor cortex will fire patterns that are specific to the patient’s thoughts.
When a paralyzed patient such as Gorham thinks about trying to move their arms or legs, the motor cortex will fire patterns that are specific to the patient’s thoughts. This pattern is detected by the Stentrode and relayed to a computer that learns to associate this pattern with the patient’s physical movements. The computer recognizes thoughts about kicking, making a fist and other movements as signals for clicking a mouse or pushing certain letters on a keyboard. An additional eye-tracking device controls the movement of the computer cursor.
The process works on a letter by letter basis. That’s why longer and more nuanced responses often involve some trial and error. “I have been using this for about two years, and I enjoy the sessions,” Gorham typed during our chat session. Zafar Faraz, field clinical engineer at Synchron, sat next to Gorham, providing help when required. Gorham had suffered without internet access, but now he looks forward to surfing the web and playing video games.

Gorham, age 63, has been enjoying Stentrode sessions for about two years.
Rodeny Dekker
The BCI revolution
In the summer of 2021, Synchron became the first company to receive the FDA’s Investigational Device Exemption, which allows research trials on the Stentrode in human patients. This past summer, the company, together with scientists from Icahn School of Medicine at Mount Sinai and the Neurology and Neurosurgery Department at Utrecht University, published a paper offering a framework for how to develop BCIs for patients with severe paralysis – those who can't use their upper limbs to type or use digital devices.
Three months ago, Synchron announced the enrollment of six patients in a study called COMMAND based in the U.S. The company will seek approval next year from the FDA to make the Stentrode available for sale commercially. Meanwhile, other companies are making progress in the field of BCIs. In August, Neuralink announced a $280 million financing round, the biggest fundraiser yet in the field. Last December, Synchron announced a $75 million financing round. “One thing I can promise you, in five years from now, we’re not going to be where we are today. We're going to be in a very different place,” says Elad I. Levy, professor of neurosurgery and radiology at State University of New York in Buffalo.
The risk of hacking exists, always. Cybercriminals, for example, might steal sensitive personal data for financial reasons, blackmailing, or to spread malware to other connected devices while extremist groups could potentially hack BCIs to manipulate individuals into supporting their causes or carrying out actions on their behalf.
“The prospect of bestowing individuals with paralysis a renewed avenue for communication and motor functionality is a step forward in neurotech,” says Hayley Nelson, a neuroscientist and founder of The Academy of Cognitive and Behavioral Neuroscience. “It is an exciting breakthrough in a world of devastating, scary diseases,” says Neil McArthur, a professor of philosophy and director of the Centre for Professional and Applied Ethics at the University of Manitoba. “To connect with the world when you are trapped inside your body is incredible.”
While the benefits for the paraplegic community are promising, the Stentrode’s long-term effectiveness and overall impact needs more research on safety. “Potential risks like inflammation, damage to neural tissue, or unexpected shifts in synaptic transmission due to the implant warrant thorough exploration,” Nelson says.
There are also concens about data privacy concerns and the policies of companies to safeguard information processed through BCIs. “Often, Big Tech is ahead of the regulators because the latter didn’t envisage such a turn of events...and companies take advantage of the lack of legal framework to push forward,” McArthur says. Hacking is another risk. Cybercriminals could steal sensitive personal data for financial reasons, blackmailing, or to spread malware to other connected devices. Extremist groups could potentially hack BCIs to manipulate individuals into supporting their causes or carrying out actions on their behalf.
“We have to protect patient identity, patient safety and patient integrity,” Levy says. “In the same way that we protect our phones or computers from hackers, we have to stay ahead with anti-hacking software.” Even so, Levy thinks the anticipated benefits for the quadriplegic community outweigh the potential risks. “We are on the precipice of an amazing technology. In the future, we would be able to connect patients to peripheral devices that enhance their quality of life.”
In the near future, the Stentrode could enable patients to use the Stentrode to activate their wheelchairs, iPods or voice modulators. Synchron's focus is on using its BCI to help patients with significant mobility restrictions—not to enhance the lives of healthy people without any illnesses. Levy says we are not prepared for the implications of endowing people with superpowers.
I wondered what Gorham thought about that. “Pardon my question, but do you feel like you have sort of transcended human nature, being the first in a big line of cybernetic people doing marvelous things with their mind only?” was my last question to Gorham.
A slight smile formed on his lips. In less than a minute, he typed: “I do a little.”
Leading XPRIZE Healthspan and Beating Negativity with Dr. Peter Diamandis
XPRIZE founder and chairman Peter Diamandis launches XPRIZE Healthspan at an event on November 29.
A new competition by the XPRIZE Foundation is offering $101 million to researchers who discover therapies that give a boost to people aged 65-80 so their bodies perform more like when they were middle-aged.
For today’s podcast episode, I talked with Dr. Peter Diamandis, XPRIZE’s founder and executive chairman. Under Peter’s leadership, XPRIZE has launched 27 previous competitions with over $300 million in prize purses. The latest contest aims to enhance healthspan, or the period of life when older people can play with their grandkids without any restriction, disability or disease. Such breakthroughs could help prevent chronic diseases that are closely linked to aging. These illnesses are costly to manage and threaten to overwhelm the healthcare system, as the number of Americans over age 65 is rising fast.
In this competition, called XPRIZE Healthspan, multiple awards are available, depending on what’s achieved, with support from the nonprofit Hevolution Foundation and Chip Wilson, the founder of Lululemon and nonprofit SOLVE FSHD. The biggest prize, $81 million, is for improvements in cognition, muscle and immunity by 20 years. An improvement of 15 years will net $71 million, and 10 years will net $61 million.
In our conversation for this episode, Peter talks about his plans for XPRIZE Healthspan and why exponential technologies make the current era - even with all of its challenges - the most exciting time in human history. We discuss the best mental outlook that supports a person in becoming truly innovative, as well as the downsides of too much risk aversion. We talk about how to overcome the negativity bias in ourselves and in mainstream media, how Peter has shifted his own mindset to become more positive over the years, how to inspire a culture of innovation, Peter’s personal recommendations for lifestyle strategies to live longer and healthier, the innovations we can expect in various fields by 2030, the future of education and the importance of democratizing tech and innovation.
In addition to Peter’s pioneering leadership of XPRIZE, he is also the Executive Founder of Singularity University. In 2014, he was named by Fortune as one of the “World’s 50 Greatest Leaders.” As an entrepreneur, he’s started over 25 companies in the areas of health-tech, space, venture capital and education. He’s Co-founder and Vice-Chairman of two public companies, Celularity and Vaxxinity, plus being Co-founder & Chairman of Fountain Life, a fully-integrated platform delivering predictive, preventative, personalized and data-driven health. He also serves as Co-founder of BOLD Capital Partners, a venture fund with a half-billion dollars under management being invested in exponential technologies and longevity companies. Peter is a New York Times Bestselling author of four books, noted during our conversation and in the show notes of this episode. He has degrees in molecular genetics and aerospace engineering from MIT and holds an M.D. from Harvard Medical School.
Show links
- Peter Diamandis bio
- New XPRIZE Healthspan
- Peter Diamandis books
- 27 XPRIZE competitions and counting
- Life Force by Peter Diamandis and Tony Robbins
- Peter Diamandis Twitter
- Longevity Insider newsletter – AI identifies the news
- Peter Diamandis Longevity Handbook
- Hevolution funding for longevity

XPRIZE Founder Peter Diamandis speaks with Mehmoud Khan, CEO of Hevolution Foundation, at the launch of XPRIZE Healthspan.
Hevolution Foundation


