Artificial Wombs Are Getting Closer to Reality for Premature Babies
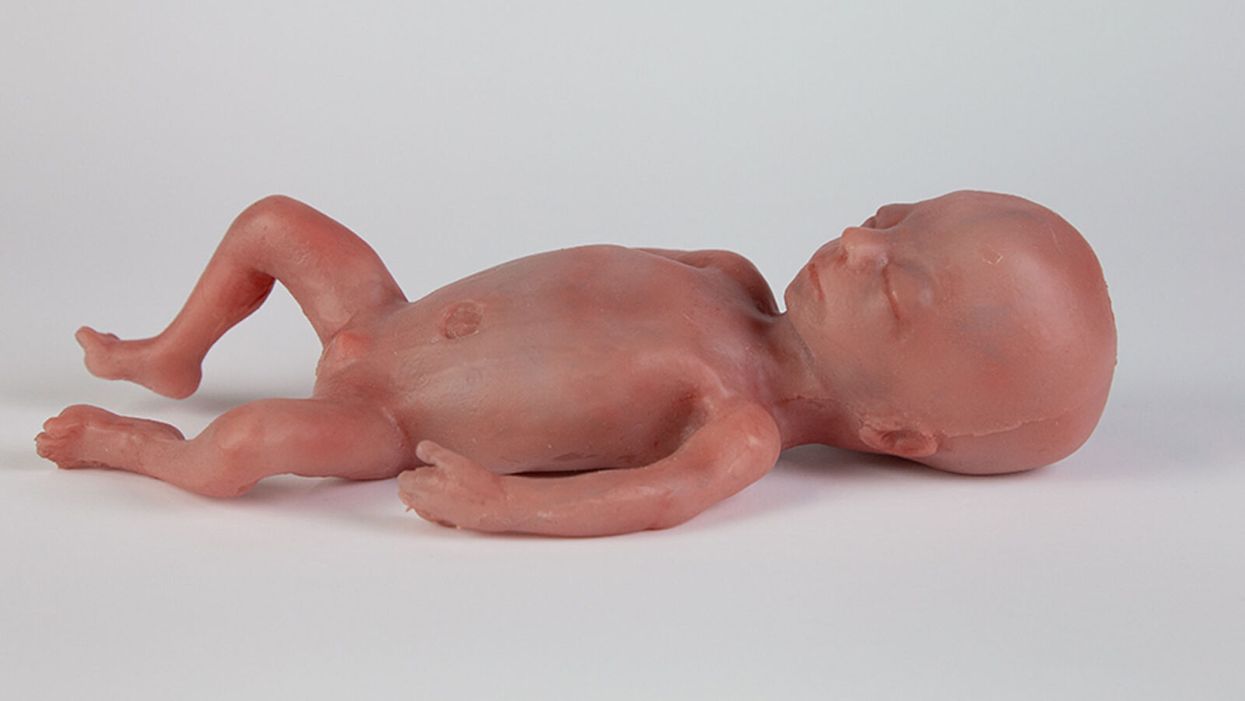
A mannequin of a 24-week-old fetus replicated from MR imaging. Created by: Juliette van Haren, Mark Thielen, Jasper Sterk, Chet Bangaru, and Frank Delbressine, Department of Industrial Design, Eindhoven University of Technology.
In 2017, researchers at the Children's Hospital of Philadelphia grew extremely preterm lambs from hairless to fluffy inside a "biobag," a dark, fluid-filled bag designed to mimic a mother's womb.
"There could be quite a lot of infants that would benefit from artificial womb technologies."
This happened over the course of a month, across a delicate period of fetal development that scientists consider the "edge of viability" for survival at birth.
In 2019, Australian and Japanese scientists repeated the success of keeping extremely premature lambs inside an artificial womb environment until they were ready to survive on their own. Those researchers are now developing a treatment strategy for infants born at "the hard limit of viability," between 20 and 23 weeks of gestation. At the same time, Dutch researchers are going so far as to replicate the sound of a mother's heartbeat inside a biobag. These developments signal exciting times ahead--with a touch of science fiction--for artificial womb technologies. But is there a catch?
"There could be quite a lot of infants that would benefit from artificial womb technologies," says Josephine Johnston, a bioethicist and lawyer at The Hastings Center, an independent bioethics research institute in New York. "These technologies can decrease morbidity and mortality for infants at the edge of viability and help them survive without significant damage to the lungs or other problems," she says.
It is a viewpoint shared by Frans van de Vosse, leader of the Cardiovascular Biomechanics research group at Eindhoven University of Technology in the Netherlands. He participates in a university project that recently received more than $3 million in funding from the E.U. to produce a prototype artificial womb for preterm babies between 24 and 28 weeks of gestation by 2024.
The Eindhoven design comes with a fluid-based environment, just like that of the natural womb, where the baby receives oxygen and nutrients through an artificial placenta that is connected to the baby's umbilical cord. "With current incubators, when a respiratory device delivers oxygen into the lungs in order for the baby to breathe, you may harm preterm babies because their lungs are not yet mature for that," says van de Vosse. "But when the lungs are under water, then they can develop, they can mature, and the baby will receive the oxygen through the umbilical cord, just like in the natural womb," he says.
His research team is working to achieve the "perfectly natural" artificial womb based on strict mathematical models and calculations, van de Vosse says. They are even employing 3D printing technology to develop the wombs and artificial babies to test in them--the mannequins, as van de Vosse calls them. These mannequins are being outfitted with sensors that can replicate the environment a fetus experiences inside a mother's womb, including the soothing sound of her heartbeat.
"The Dutch study's artificial womb design is slightly different from everything else we have seen as it encourages a gestateling to experience the kind of intimacy that a fetus does in pregnancy," says Elizabeth Chloe Romanis, an assistant professor in biolaw at Durham Law School in the U.K. But what is a "gestateling" anyway? It's a term Romanis has coined to describe neither a fetus nor a newborn, but an in-between artificial stage.
"Because they aren't born, they are not neonates," Romanis explains. "But also, they are not inside a pregnant person's body, so they are not fetuses. In an artificial womb the fetus is still gestating, hence why I call it gestateling."
The terminology is not just a semantic exercise to lend a name to what medical dictionaries haven't yet defined. "Gestatelings might have a slightly different psychology," says Romanis. "A fetus inside a mother's womb interacts with the mother. A neonate has some kind of self-sufficiency in terms of physiology. But the gestateling doesn't do either of those things," she says, urging us to be mindful of the still-obscure effects that experiencing early life as a gestateling might have on future humans. Psychology aside, there are also legal repercussions.
The Universal Declaration of Human Rights proclaims the "inalienable rights which everyone is entitled to as a human being," with "everyone" including neonates. However, such a legal umbrella is absent when it comes to fetuses, which have no rights under the same declaration. "We might need a new legal category for a gestateling," concludes Romanis.
But not everyone agrees. "However well-meaning, a new legal category would almost certainly be used to further erode the legality of abortion in countries like the U.S.," says Johnston.
The "abortion war" in the U.S. has risen to a crescendo since 2019, when states like Missouri, Mississippi, Kentucky, Louisiana and Georgia passed so-called "fetal heartbeat bills," which render an abortion illegal once a fetal heartbeat is detected. The situation is only bound to intensify now that Justice Ruth Bader Ginsburg, one of the Supreme Court's fiercest champions for abortion rights, has passed away. If President Trump appoints Ginsburg's replacement, he will probably grant conservatives on the Court the votes needed to revoke or weaken Roe v. Wade, the milestone decision of 1973 that established women's legal right to an abortion.
"A gestateling with intermediate status would almost certainly be considered by some in the U.S. (including some judges) to have at least certain legal rights, likely including right-to-life," says Johnston. This would enable a fetus on the edge of viability to make claims on the mother, and lead either to a shortening of the window in which abortion is legal—or a practice of denying abortion altogether. Instead, Johnston predicts, doctors might offer to transfer the fetus to an artificial womb for external gestation as a new standard of care.
But the legal conundrum does not stop there. The viability threshold is an estimate decided by medical professionals based on the clinical evidence and the technology available. It is anything but static. In the 1970s when Roe v. Wade was decided, for example, a fetus was considered legally viable starting at 28 weeks. Now, with improved technology and medical management, "the hard limit today is probably 20 or 21 weeks," says Matthew Kemp, associate professor at the University of Western Australia and one of the Australian-Japanese artificial womb project's senior researchers.
The changing threshold can result in situations where lots of people invested in the decision disagree. "Those can be hard decisions, but they are case-by-case decisions that families make or parents make with the key providers to determine when to proceed and when to let the infant die. Usually, it's a shared decision where the parents have the final say," says Johnston. But this isn't always the case.
On May 9th 2016, a boy named Alfie Evans was born in Liverpool, UK. Suffering seizures a few months after his birth, Alfie was diagnosed with an unknown neurodegenerative disorder and soon went into a semi-vegetative state, which lasted for more than a year. Alfie's medical team decided to withdraw his ventilation support, suggesting further treatment was unlawful and inhumane, but his parents wanted permission to fly him to a hospital in Rome and attempt to prolong his life there. In the end, the case went all the way up to the Supreme Court, which ruled that doctors could stop providing life support for Alfie, saying that the child required "peace, quiet and privacy." What happened to little Alfie raised huge publicity in the UK and pointedly highlighted the dilemma of whether parents or doctors should have the final say in the fate of a terminally-ill child in life-support treatment.
"In a few years from now, women who cannot get pregnant because of uterine infertility will be able to have a fully functional uterus made from their own tissue."
Alfie was born and, thus had legal rights, yet legal and ethical mayhem arose out of his case. When it comes to gestatelings, the scenarios will be even more complicated, says Romanis. "I think there's a really big question about who has parental rights and who doesn't," she says. "The assisted reproductive technology (ART) law in the U.K. hasn't been updated since 2008....It certainly needs an update when you think about all the things we have done since [then]."
This June, for instance, scientists from the Wake Forest Institute for Regenerative Medicine in North Carolina published research showing that they could take a small sample of tissue from a rabbit's uterus and create a bioengineered uterus, which then supported both fertilization and normal pregnancy like a natural uterus does.
"In [a number of] years from now, women who cannot get pregnant because of uterine infertility will be able to have a fully functional uterus made from their own tissue," says Dr. Anthony Atala, the Institute's director and a pioneer in regenerative medicine. These bioengineered uteri will eventually be covered by insurance, Atala expects. But when it comes to artificial wombs that externally gestate premature infants, will all mothers have equal access?
Medical reports have already shown racial and ethnic disparities in infertility treatments and access to assisted reproductive technologies. Costs on average total $12,400 per cycle of treatment and may require several cycles to achieve a live birth. "There's no indication that artificial wombs would be treated any differently. That's what we see with almost every expensive new medical technology," says Johnston. In a much more dystopian future, there is even a possibility that inequity in healthcare might create disturbing chasms in how women of various class levels bear children. Romanis asks us to picture the following scenario:
We live in a world where artificial wombs have become mainstream. Most women choose to end their pregnancies early and transfer their gestatelings to the care of machines. After a while, insurers deem full-term pregnancy and childbirth a risky non-necessity, and are lobbying to stop covering them altogether. Wealthy white women continue opting out of their third trimesters (at a high cost), since natural pregnancy has become a substandard route for poorer women. Those women are strongly judged for any behaviors that could risk their fetus's health, in contrast with the machine's controlled environment. "Why are you having a coffee during your pregnancy?" critics might ask. "Why are you having a glass of red wine? If you can't be perfect, why don't you have it the artificial way?"
Problem is, even if they want to, they won't be able to afford it.
In a more sanguine version, however, the artificial wombs are only used in cases of prematurity as a life-saving medical intervention rather than as a lifestyle accommodation. The 15 million babies who are born prematurely each year and may face serious respiratory, cardiovascular, visual and hearing problems, as well as learning disabilities, instead continue their normal development in artificial wombs. After lots of deliberation, insurers agree to bear the cost of external wombs because they are cheaper than a lifetime of medical care for a disabled or diseased person. This enables racial and ethnic minority women, who make up the majority of women giving premature birth, to access the technology.
Even extremely premature babies, those babies (far) below the threshold of 28 weeks of gestation, half of which die, could now discover this thing called life. In this scenario, as the Australian researcher Kemp says, we are simply giving a good shot at healthy, long-term survival to those who were unfortunate enough to start too soon.
Have You Heard of the Best Sport for Brain Health?
In this week's Friday Five, research points to this brain healthiest of sports. Plus, the natural way to reprogram cells to a younger state, the network that could underlie many different mental illnesses, and a new test could diagnose autism in newborns. Plus, scientists 3D print an ear and attach it to woman
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Here are the promising studies covered in this week's Friday Five:
- Reprogram cells to a younger state
- Pick up this sport for brain health
- Do all mental illnesses have the same underlying cause?
- New test could diagnose autism in newborns
- Scientists 3D print an ear and attach it to woman
Can blockchain help solve the Henrietta Lacks problem?
Marielle Gross, a professor at the University of Pittsburgh, shows patients a new app that tracks how their samples are used during biomedical research.
Science has come a long way since Henrietta Lacks, a Black woman from Baltimore, succumbed to cervical cancer at age 31 in 1951 -- only eight months after her diagnosis. Since then, research involving her cancer cells has advanced scientific understanding of the human papilloma virus, polio vaccines, medications for HIV/AIDS and in vitro fertilization.
Today, the World Health Organization reports that those cells are essential in mounting a COVID-19 response. But they were commercialized without the awareness or permission of Lacks or her family, who have filed a lawsuit against a biotech company for profiting from these “HeLa” cells.
While obtaining an individual's informed consent has become standard procedure before the use of tissues in medical research, many patients still don’t know what happens to their samples. Now, a new phone-based app is aiming to change that.
Tissue donors can track what scientists do with their samples while safeguarding privacy, through a pilot program initiated in October by researchers at the Johns Hopkins Berman Institute of Bioethics and the University of Pittsburgh’s Institute for Precision Medicine. The program uses blockchain technology to offer patients this opportunity through the University of Pittsburgh's Breast Disease Research Repository, while assuring that their identities remain anonymous to investigators.
A blockchain is a digital, tamper-proof ledger of transactions duplicated and distributed across a computer system network. Whenever a transaction occurs with a patient’s sample, multiple stakeholders can track it while the owner’s identity remains encrypted. Special certificates called “nonfungible tokens,” or NFTs, represent patients’ unique samples on a trusted and widely used blockchain that reinforces transparency.
Blockchain could be used to notify people if cancer researchers discover that they have certain risk factors.
“Healthcare is very data rich, but control of that data often does not lie with the patient,” said Julius Bogdan, vice president of analytics for North America at the Healthcare Information and Management Systems Society (HIMSS), a Chicago-based global technology nonprofit. “NFTs allow for the encapsulation of a patient’s data in a digital asset controlled by the patient.” He added that this technology enables a more secure and informed method of participating in clinical and research trials.
Without this technology, de-identification of patients’ samples during biomedical research had the unintended consequence of preventing them from discovering what researchers find -- even if that data could benefit their health. A solution was urgently needed, said Marielle Gross, assistant professor of obstetrics, gynecology and reproductive science and bioethics at the University of Pittsburgh School of Medicine.
“A researcher can learn something from your bio samples or medical records that could be life-saving information for you, and they have no way to let you or your doctor know,” said Gross, who is also an affiliate assistant professor at the Berman Institute. “There’s no good reason for that to stay the way that it is.”
For instance, blockchain could be used to notify people if cancer researchers discover that they have certain risk factors. Gross estimated that less than half of breast cancer patients are tested for mutations in BRCA1 and BRCA2 — tumor suppressor genes that are important in combating cancer. With normal function, these genes help prevent breast, ovarian and other cells from proliferating in an uncontrolled manner. If researchers find mutations, it’s relevant for a patient’s and family’s follow-up care — and that’s a prime example of how this newly designed app could play a life-saving role, she said.
Liz Burton was one of the first patients at the University of Pittsburgh to opt for the app -- called de-bi, which is short for decentralized biobank -- before undergoing a mastectomy for early-stage breast cancer in November, after it was diagnosed on a routine mammogram. She often takes part in medical research and looks forward to tracking her tissues.
“Anytime there’s a scientific experiment or study, I’m quick to participate -- to advance my own wellness as well as knowledge in general,” said Burton, 49, a life insurance service representative who lives in Carnegie, Pa. “It’s my way of contributing.”

Liz Burton was one of the first patients at the University of Pittsburgh to opt for the app before undergoing a mastectomy for early-stage breast cancer.
Liz Burton
The pilot program raises the issue of what investigators may owe study participants, especially since certain populations, such as Black and indigenous peoples, historically were not treated in an ethical manner for scientific purposes. “It’s a truly laudable effort,” Tamar Schiff, a postdoctoral fellow in medical ethics at New York University’s Grossman School of Medicine, said of the endeavor. “Research participants are beautifully altruistic.”
Lauren Sankary, a bioethicist and associate director of the neuroethics program at Cleveland Clinic, agrees that the pilot program provides increased transparency for study participants regarding how scientists use their tissues while acknowledging individuals’ contributions to research.
However, she added, “it may require researchers to develop a process for ongoing communication to be responsive to additional input from research participants.”
Peter H. Schwartz, professor of medicine and director of Indiana University’s Center for Bioethics in Indianapolis, said the program is promising, but he wonders what will happen if a patient has concerns about a particular research project involving their tissues.
“I can imagine a situation where a patient objects to their sample being used for some disease they’ve never heard about, or which carries some kind of stigma like a mental illness,” Schwartz said, noting that researchers would have to evaluate how to react. “There’s no simple answer to those questions, but the technology has to be assessed with an eye to the problems it could raise.”
To truly make a difference, blockchain must enable broad consent from patients, not just de-identification.
As a result, researchers may need to factor in how much information to share with patients and how to explain it, Schiff said. There are also concerns that in tracking their samples, patients could tell others what they learned before researchers are ready to publicly release this information. However, Bogdan, the vice president of the HIMSS nonprofit, believes only a minimal study identifier would be stored in an NFT, not patient data, research results or any type of proprietary trial information.
Some patients may be confused by blockchain and reluctant to embrace it. “The complexity of NFTs may prevent the average citizen from capitalizing on their potential or vendors willing to participate in the blockchain network,” Bogdan said. “Blockchain technology is also quite costly in terms of computational power and energy consumption, contributing to greenhouse gas emissions and climate change.”
In addition, this nascent, groundbreaking technology is immature and vulnerable to data security flaws, disputes over intellectual property rights and privacy issues, though it does offer baseline protections to maintain confidentiality. To truly make a difference, blockchain must enable broad consent from patients, not just de-identification, said Robyn Shapiro, a bioethicist and founding attorney at Health Sciences Law Group near Milwaukee.
The Henrietta Lacks story is a prime example, Shapiro noted. During her treatment for cervical cancer at Johns Hopkins, Lacks’s tissue was de-identified (albeit not entirely, because her cell line, HeLa, bore her initials). After her death, those cells were replicated and distributed for important and lucrative research and product development purposes without her knowledge or consent.
Nonetheless, Shapiro thinks that the initiative by the University of Pittsburgh and Johns Hopkins has potential to solve some ethical challenges involved in research use of biospecimens. “Compared to the system that allowed Lacks’s cells to be used without her permission, Shapiro said, “blockchain technology using nonfungible tokens that allow patients to follow their samples may enhance transparency, accountability and respect for persons who contribute their tissue and clinical data for research.”
Read more about laws that have prevented people from the rights to their own cells.

