Artificial Wombs Are Getting Closer to Reality for Premature Babies
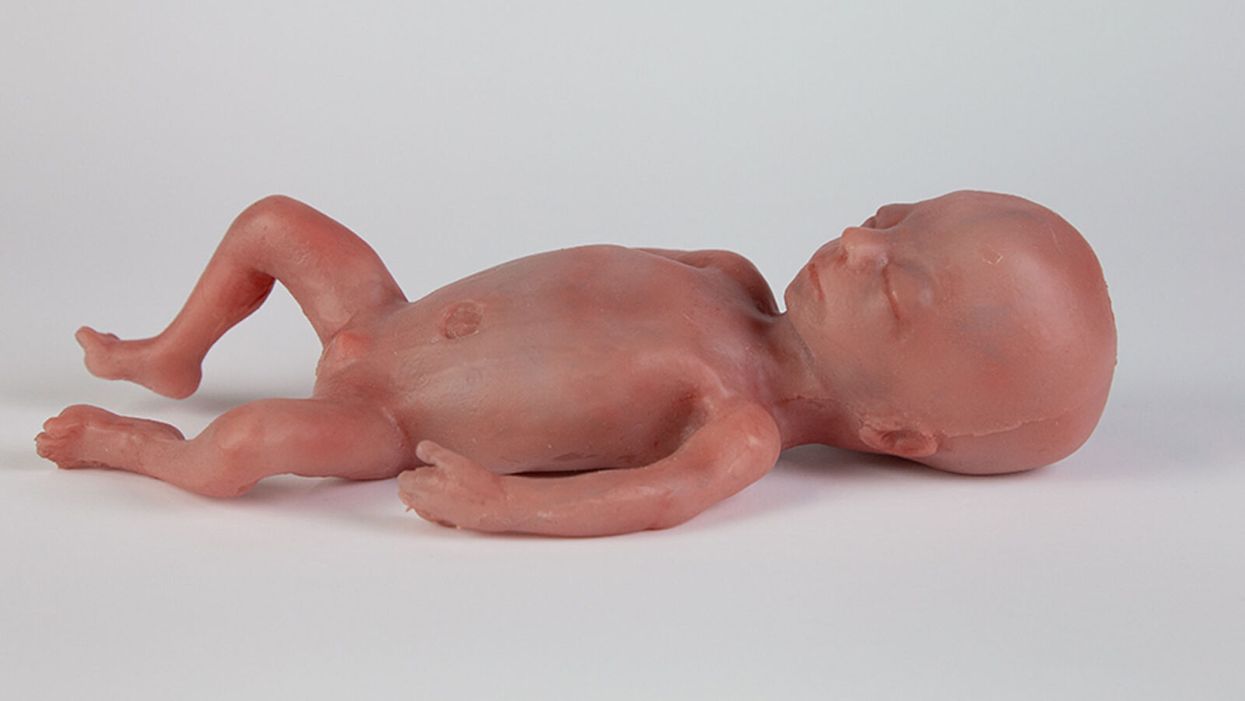
A mannequin of a 24-week-old fetus replicated from MR imaging. Created by: Juliette van Haren, Mark Thielen, Jasper Sterk, Chet Bangaru, and Frank Delbressine, Department of Industrial Design, Eindhoven University of Technology.
In 2017, researchers at the Children's Hospital of Philadelphia grew extremely preterm lambs from hairless to fluffy inside a "biobag," a dark, fluid-filled bag designed to mimic a mother's womb.
"There could be quite a lot of infants that would benefit from artificial womb technologies."
This happened over the course of a month, across a delicate period of fetal development that scientists consider the "edge of viability" for survival at birth.
In 2019, Australian and Japanese scientists repeated the success of keeping extremely premature lambs inside an artificial womb environment until they were ready to survive on their own. Those researchers are now developing a treatment strategy for infants born at "the hard limit of viability," between 20 and 23 weeks of gestation. At the same time, Dutch researchers are going so far as to replicate the sound of a mother's heartbeat inside a biobag. These developments signal exciting times ahead--with a touch of science fiction--for artificial womb technologies. But is there a catch?
"There could be quite a lot of infants that would benefit from artificial womb technologies," says Josephine Johnston, a bioethicist and lawyer at The Hastings Center, an independent bioethics research institute in New York. "These technologies can decrease morbidity and mortality for infants at the edge of viability and help them survive without significant damage to the lungs or other problems," she says.
It is a viewpoint shared by Frans van de Vosse, leader of the Cardiovascular Biomechanics research group at Eindhoven University of Technology in the Netherlands. He participates in a university project that recently received more than $3 million in funding from the E.U. to produce a prototype artificial womb for preterm babies between 24 and 28 weeks of gestation by 2024.
The Eindhoven design comes with a fluid-based environment, just like that of the natural womb, where the baby receives oxygen and nutrients through an artificial placenta that is connected to the baby's umbilical cord. "With current incubators, when a respiratory device delivers oxygen into the lungs in order for the baby to breathe, you may harm preterm babies because their lungs are not yet mature for that," says van de Vosse. "But when the lungs are under water, then they can develop, they can mature, and the baby will receive the oxygen through the umbilical cord, just like in the natural womb," he says.
His research team is working to achieve the "perfectly natural" artificial womb based on strict mathematical models and calculations, van de Vosse says. They are even employing 3D printing technology to develop the wombs and artificial babies to test in them--the mannequins, as van de Vosse calls them. These mannequins are being outfitted with sensors that can replicate the environment a fetus experiences inside a mother's womb, including the soothing sound of her heartbeat.
"The Dutch study's artificial womb design is slightly different from everything else we have seen as it encourages a gestateling to experience the kind of intimacy that a fetus does in pregnancy," says Elizabeth Chloe Romanis, an assistant professor in biolaw at Durham Law School in the U.K. But what is a "gestateling" anyway? It's a term Romanis has coined to describe neither a fetus nor a newborn, but an in-between artificial stage.
"Because they aren't born, they are not neonates," Romanis explains. "But also, they are not inside a pregnant person's body, so they are not fetuses. In an artificial womb the fetus is still gestating, hence why I call it gestateling."
The terminology is not just a semantic exercise to lend a name to what medical dictionaries haven't yet defined. "Gestatelings might have a slightly different psychology," says Romanis. "A fetus inside a mother's womb interacts with the mother. A neonate has some kind of self-sufficiency in terms of physiology. But the gestateling doesn't do either of those things," she says, urging us to be mindful of the still-obscure effects that experiencing early life as a gestateling might have on future humans. Psychology aside, there are also legal repercussions.
The Universal Declaration of Human Rights proclaims the "inalienable rights which everyone is entitled to as a human being," with "everyone" including neonates. However, such a legal umbrella is absent when it comes to fetuses, which have no rights under the same declaration. "We might need a new legal category for a gestateling," concludes Romanis.
But not everyone agrees. "However well-meaning, a new legal category would almost certainly be used to further erode the legality of abortion in countries like the U.S.," says Johnston.
The "abortion war" in the U.S. has risen to a crescendo since 2019, when states like Missouri, Mississippi, Kentucky, Louisiana and Georgia passed so-called "fetal heartbeat bills," which render an abortion illegal once a fetal heartbeat is detected. The situation is only bound to intensify now that Justice Ruth Bader Ginsburg, one of the Supreme Court's fiercest champions for abortion rights, has passed away. If President Trump appoints Ginsburg's replacement, he will probably grant conservatives on the Court the votes needed to revoke or weaken Roe v. Wade, the milestone decision of 1973 that established women's legal right to an abortion.
"A gestateling with intermediate status would almost certainly be considered by some in the U.S. (including some judges) to have at least certain legal rights, likely including right-to-life," says Johnston. This would enable a fetus on the edge of viability to make claims on the mother, and lead either to a shortening of the window in which abortion is legal—or a practice of denying abortion altogether. Instead, Johnston predicts, doctors might offer to transfer the fetus to an artificial womb for external gestation as a new standard of care.
But the legal conundrum does not stop there. The viability threshold is an estimate decided by medical professionals based on the clinical evidence and the technology available. It is anything but static. In the 1970s when Roe v. Wade was decided, for example, a fetus was considered legally viable starting at 28 weeks. Now, with improved technology and medical management, "the hard limit today is probably 20 or 21 weeks," says Matthew Kemp, associate professor at the University of Western Australia and one of the Australian-Japanese artificial womb project's senior researchers.
The changing threshold can result in situations where lots of people invested in the decision disagree. "Those can be hard decisions, but they are case-by-case decisions that families make or parents make with the key providers to determine when to proceed and when to let the infant die. Usually, it's a shared decision where the parents have the final say," says Johnston. But this isn't always the case.
On May 9th 2016, a boy named Alfie Evans was born in Liverpool, UK. Suffering seizures a few months after his birth, Alfie was diagnosed with an unknown neurodegenerative disorder and soon went into a semi-vegetative state, which lasted for more than a year. Alfie's medical team decided to withdraw his ventilation support, suggesting further treatment was unlawful and inhumane, but his parents wanted permission to fly him to a hospital in Rome and attempt to prolong his life there. In the end, the case went all the way up to the Supreme Court, which ruled that doctors could stop providing life support for Alfie, saying that the child required "peace, quiet and privacy." What happened to little Alfie raised huge publicity in the UK and pointedly highlighted the dilemma of whether parents or doctors should have the final say in the fate of a terminally-ill child in life-support treatment.
"In a few years from now, women who cannot get pregnant because of uterine infertility will be able to have a fully functional uterus made from their own tissue."
Alfie was born and, thus had legal rights, yet legal and ethical mayhem arose out of his case. When it comes to gestatelings, the scenarios will be even more complicated, says Romanis. "I think there's a really big question about who has parental rights and who doesn't," she says. "The assisted reproductive technology (ART) law in the U.K. hasn't been updated since 2008....It certainly needs an update when you think about all the things we have done since [then]."
This June, for instance, scientists from the Wake Forest Institute for Regenerative Medicine in North Carolina published research showing that they could take a small sample of tissue from a rabbit's uterus and create a bioengineered uterus, which then supported both fertilization and normal pregnancy like a natural uterus does.
"In [a number of] years from now, women who cannot get pregnant because of uterine infertility will be able to have a fully functional uterus made from their own tissue," says Dr. Anthony Atala, the Institute's director and a pioneer in regenerative medicine. These bioengineered uteri will eventually be covered by insurance, Atala expects. But when it comes to artificial wombs that externally gestate premature infants, will all mothers have equal access?
Medical reports have already shown racial and ethnic disparities in infertility treatments and access to assisted reproductive technologies. Costs on average total $12,400 per cycle of treatment and may require several cycles to achieve a live birth. "There's no indication that artificial wombs would be treated any differently. That's what we see with almost every expensive new medical technology," says Johnston. In a much more dystopian future, there is even a possibility that inequity in healthcare might create disturbing chasms in how women of various class levels bear children. Romanis asks us to picture the following scenario:
We live in a world where artificial wombs have become mainstream. Most women choose to end their pregnancies early and transfer their gestatelings to the care of machines. After a while, insurers deem full-term pregnancy and childbirth a risky non-necessity, and are lobbying to stop covering them altogether. Wealthy white women continue opting out of their third trimesters (at a high cost), since natural pregnancy has become a substandard route for poorer women. Those women are strongly judged for any behaviors that could risk their fetus's health, in contrast with the machine's controlled environment. "Why are you having a coffee during your pregnancy?" critics might ask. "Why are you having a glass of red wine? If you can't be perfect, why don't you have it the artificial way?"
Problem is, even if they want to, they won't be able to afford it.
In a more sanguine version, however, the artificial wombs are only used in cases of prematurity as a life-saving medical intervention rather than as a lifestyle accommodation. The 15 million babies who are born prematurely each year and may face serious respiratory, cardiovascular, visual and hearing problems, as well as learning disabilities, instead continue their normal development in artificial wombs. After lots of deliberation, insurers agree to bear the cost of external wombs because they are cheaper than a lifetime of medical care for a disabled or diseased person. This enables racial and ethnic minority women, who make up the majority of women giving premature birth, to access the technology.
Even extremely premature babies, those babies (far) below the threshold of 28 weeks of gestation, half of which die, could now discover this thing called life. In this scenario, as the Australian researcher Kemp says, we are simply giving a good shot at healthy, long-term survival to those who were unfortunate enough to start too soon.
The future of non-hormonal birth control: Antibodies can stop sperm in their tracks
Many women want non-hormonal birth control. A 22-year-old's findings were used to launch a company that could, within the decade, bring a new kind of contraceptive to the marketplace.
Unwanted pregnancy can now be added to the list of preventions that antibodies may be fighting in the near future. For decades, really since the 1980s, engineered monoclonal antibodies have been knocking out invading germs — preventing everything from cancer to COVID. Sperm, which have some of the same properties as germs, may be next.
Not only is there an unmet need on the market for alternatives to hormonal contraceptives, the genesis for the original research was personal for the then 22-year-old scientist who led it. Her findings were used to launch a company that could, within the decade, bring a new kind of contraceptive to the marketplace.
The genesis
It’s Suruchi Shrestha’s research — published in Science Translational Medicine in August 2021 and conducted as part of her dissertation while she was a graduate student at the University of North Carolina at Chapel Hill — that could change the future of contraception for many women worldwide. According to a Guttmacher Institute report, in the U.S. alone, there were 46 million sexually active women of reproductive age (15–49) who did not want to get pregnant in 2018. With the overturning of Roe v. Wade this year, Shrestha’s research could, indeed, be life changing for millions of American women and their families.
Now a scientist with NextVivo, Shrestha is not directly involved in the development of the contraceptive that is based on her research. But, back in 2016 when she was going through her own problems with hormonal contraceptives, she “was very personally invested” in her research project, Shrestha says. She was coping with a long list of negative effects from an implanted hormonal IUD. According to the Mayo Clinic, those can include severe pelvic pain, headaches, acute acne, breast tenderness, irregular bleeding and mood swings. After a year, she had the IUD removed, but it took another full year before all the side effects finally subsided; she also watched her sister suffer the “same tribulations” after trying a hormonal IUD, she says.
For contraceptive use either daily or monthly, Shrestha says, “You want the antibody to be very potent and also cheap.” That was her goal when she launched her study.
Shrestha unshelved antibody research that had been sitting idle for decades. It was in the late 80s that scientists in Japan first tried to develop anti-sperm antibodies for contraceptive use. But, 35 years ago, “Antibody production had not been streamlined as it is now, so antibodies were very expensive,” Shrestha explains. So, they shifted away from birth control, opting to focus on developing antibodies for vaccines.
Over the course of the last three decades, different teams of researchers have been working to make the antibody more effective, bringing the cost down, though it’s still expensive, according to Shrestha. For contraceptive use either daily or monthly, she says, “You want the antibody to be very potent and also cheap.” That was her goal when she launched her study.
The problem
The problem with contraceptives for women, Shrestha says, is that all but a few of them are hormone-based or have other negative side effects. In fact, some studies and reports show that millions of women risk unintended pregnancy because of medical contraindications with hormone-based contraceptives or to avoid the risks and side effects. While there are about a dozen contraceptive choices for women, there are two for men: the condom, considered 98% effective if used correctly, and vasectomy, 99% effective. Neither of these choices are hormone-based.
On the non-hormonal side for women, there is the diaphragm which is considered only 87 percent effective. It works better with the addition of spermicides — Nonoxynol-9, or N-9 — however, they are detergents; they not only kill the sperm, they also erode the vaginal epithelium. And, there’s the non-hormonal IUD which is 99% effective. However, the IUD needs to be inserted by a medical professional, and it has a number of negative side effects, including painful cramping at a higher frequency and extremely heavy or “abnormal” and unpredictable menstrual flows.
The hormonal version of the IUD, also considered 99% effective, is the one Shrestha used which caused her two years of pain. Of course, there’s the pill, which needs to be taken daily, and the birth control ring which is worn 24/7. Both cause side effects similar to the other hormonal contraceptives on the market. The ring is considered 93% effective mostly because of user error; the pill is considered 99% effective if taken correctly.
“That’s where we saw this opening or gap for women. We want a safe, non-hormonal contraceptive,” Shrestha says. Compounding the lack of good choices, is poor access to quality sex education and family planning information, according to the non-profit Urban Institute. A focus group survey suggested that the sex education women received “often lacked substance, leaving them feeling unprepared to make smart decisions about their sexual health and safety,” wrote the authors of the Urban Institute report. In fact, nearly half (45%, or 2.8 million) of the pregnancies that occur each year in the US are unintended, reports the Guttmacher Institute. Globally the numbers are similar. According to a new report by the United Nations, each year there are 121 million unintended pregnancies, worldwide.
The science
The early work on antibodies as a contraceptive had been inspired by women with infertility. It turns out that 9 to 12 percent of women who are treated for infertility have antibodies that develop naturally and work against sperm. Shrestha was encouraged that the antibodies were specific to the target — sperm — and therefore “very safe to use in women.” She aimed to make the antibodies more stable, more effective and less expensive so they could be more easily manufactured.
Since antibodies tend to stick to things that you tell them to stick to, the idea was, basically, to engineer antibodies to stick to sperm so they would stop swimming. Shrestha and her colleagues took the binding arm of an antibody that they’d isolated from an infertile woman. Then, targeting a unique surface antigen present on human sperm, they engineered a panel of antibodies with as many as six to 10 binding arms — “almost like tongs with prongs on the tongs, that bind the sperm,” explains Shrestha. “We decided to add those grabbers on top of it, behind it. So it went from having two prongs to almost 10. And the whole goal was to have so many arms binding the sperm that it clumps it” into a “dollop,” explains Shrestha, who earned a patent on her research.

Suruchi Shrestha works in the lab with a colleague. In 2016, her research on antibodies for birth control was inspired by her own experience with side effects from an implanted hormonal IUD.
UNC - Chapel Hill
The sperm stays right where it met the antibody, never reaching the egg for fertilization. Eventually, and naturally, “Our vaginal system will just flush it out,” Shrestha explains.
“She showed in her early studies that [she] definitely got the sperm immotile, so they didn't move. And that was a really promising start,” says Jasmine Edelstein, a scientist with an expertise in antibody engineering who was not involved in this research. Shrestha’s team at UNC reproduced the effect in the sheep, notes Edelstein, who works at the startup Be Biopharma. In fact, Shrestha’s anti-sperm antibodies that caused the sperm to agglutinate, or clump together, were 99.9% effective when delivered topically to the sheep’s reproductive tracts.
The future
Going forward, Shrestha thinks the ideal approach would be delivering the antibodies through a vaginal ring. “We want to use it at the source of the spark,” Shrestha says, as opposed to less direct methods, such as taking a pill. The ring would dissolve after one month, she explains, “and then you get another one.”
Engineered to have a long shelf life, the anti-sperm antibody ring could be purchased without a prescription, and women could insert it themselves, without a doctor. “That's our hope, so that it is accessible,” Shrestha says. “Anybody can just go and grab it and not worry about pregnancy or unintended pregnancy.”
Her patented research has been licensed by several biotech companies for clinical trials. A number of Shrestha’s co-authors, including her lab advisor, Sam Lai, have launched a company, Mucommune, to continue developing the contraceptives based on these antibodies.
And, results from a small clinical trial run by researchers at Boston University Chobanian & Avedisian School of Medicine show that a dissolvable vaginal film with antibodies was safe when tested on healthy women of reproductive age. That same group of researchers earlier this year received a $7.2 million grant from the National Institute of Health for further research on monoclonal antibody-based contraceptives, which have also been shown to block transmission of viruses, like HIV.
“As the costs come down, this becomes a more realistic option potentially for women,” says Edelstein. “The impact could be tremendous.”
The Friday Five: An mRNA vaccine works against cancer, new research suggests
In this week's Friday Five, an mRNA vaccine works against cancer for the first time. Plus, these cameras inside the body have an unusual source of power, a new theory for what causes aging, bacteria that could get you excited to work out, and a reason for sex differences in Alzheimer's.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Here are the promising studies covered in this week's Friday Five:
- An mRNA vaccine that works against cancer
- These cameras inside the body have an unusual source of power
- A new theory for what causes aging
- Can bacteria make you excited to work out?
- Why women get Alzheimer's more often than men