Blood Money: Paying for Convalescent Plasma to Treat COVID-19
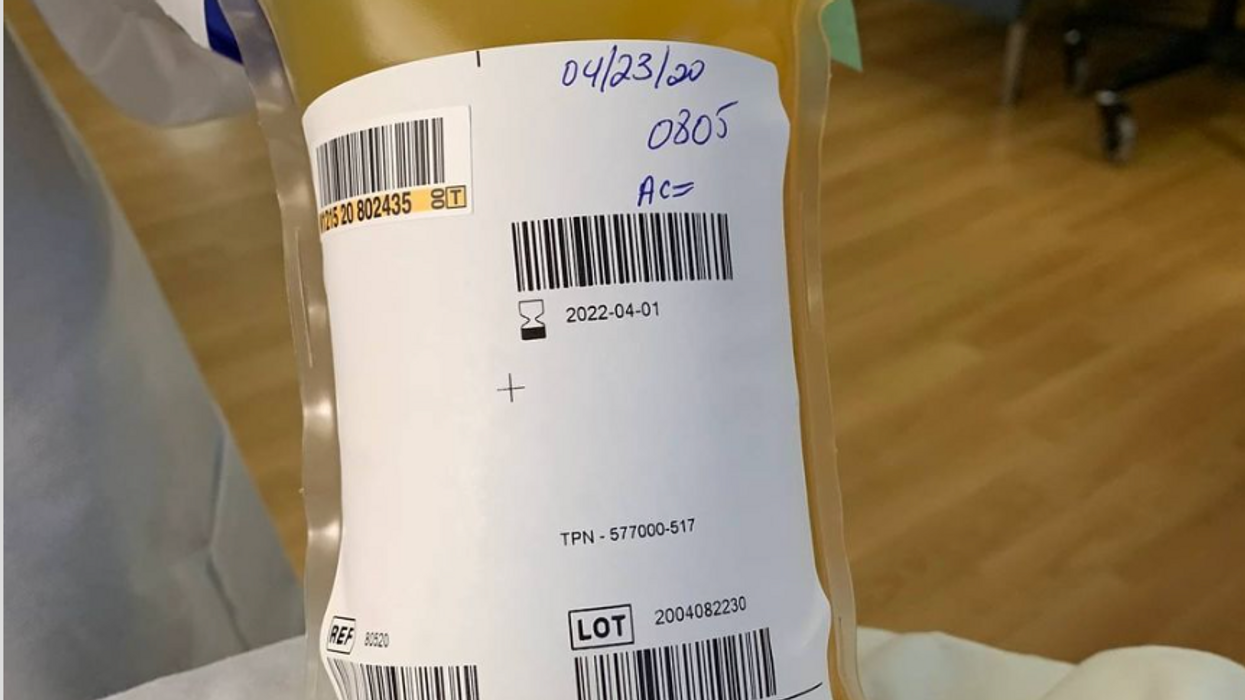
A bag of plasma that Tom Hanks donated back in April 2020 after his coronavirus infection. (He was not paid to donate.)
Convalescent plasma – first used to treat diphtheria in 1890 – has been dusted off the shelf to treat COVID-19. Does it work? Should we rely strictly on the altruism of donors or should people be paid for it?
The biologic theory is that a person who has recovered from a disease has chemicals in their blood, most likely antibodies, that contributed to their recovery, and transferring those to a person who is sick might aid their recovery. Whole blood won't work because there are too few antibodies in a single unit of blood and the body can hold only so much of it.
Plasma comprises about 55 percent of whole blood and is what's left once you take out the red blood cells that carry oxygen and the white blood cells of the immune system. Most of it is water but the rest is a complex mix of fats, salts, signaling molecules and proteins produced by the immune system, including antibodies.
A process called apheresis circulates the donors' blood through a machine that separates out the desired parts of blood and returns the rest to the donor. It takes several times the length of a regular whole blood donation to cycle through enough blood for the process. The end product is a yellowish concentration called convalescent plasma.
Recent History
It was used extensively during the great influenza epidemic off 1918 but fell out of favor with the development of antibiotics. Still, whenever a new disease emerges – SARS, MERS, Ebola, even antibiotic-resistant bacteria – doctors turn to convalescent plasma, often as a stopgap until more effective antibiotic and antiviral drugs are developed. The process is certainly safe when standard procedures for handling blood products are followed, and historically it does seem to be beneficial in at least some patients if administered early enough in the disease.
With few good treatment options for COVID-19, doctors have given convalescent plasma to more than a hundred thousand Americans and tens of thousand of people elsewhere, to mixed results. Placebo-controlled trials could give a clearer picture of plasma's value but it is difficult to enroll patients facing possible death when the flip of a coin will determine who will receive a saline solution or plasma.
And the plasma itself isn't some uniform pill stamped out in a factory, it's a natural product that is shaped by the immune history of the donor's body and its encounter not just with SARS-CoV-2 but a lifetime of exposure to different pathogens.
Researchers believe antibodies in plasma are a key factor in directly fighting the virus. But the variety and quantity of antibodies vary from donor to donor, and even over time from the same donor because once the immune system has cleared the virus from the body, it stops putting out antibodies to fight the virus. Often the quality and quantity of antibodies being given to a patient are not measured, making it somewhat hit or miss, which is why several companies have recently developed monoclonal antibodies, a single type of antibody found in blood that is effective against SARS-CoV-2 and that is multiplied in the lab for use as therapy.
Plasma may also contain other unknown factors that contribute to fighting disease, say perhaps signaling molecules that affect gene expression, which might affect the movement of immune cells, their production of antiviral molecules, or the regulation of inflammation. The complexity and lack of standardization makes it difficult to evaluate what might be working or not with a convalescent plasma treatment. Thus researchers are left with few clues about how to make it more effective.
Industrializing Plasma
Many Americans living along the border with Mexico regularly head south to purchase prescription drugs at a significant discount. Less known is the medical traffic the other way, Mexicans who regularly head north to be paid for plasma donations, which are prohibited in their country; the U.S. allows payment for plasma donations but not whole blood. A typical payment is about $35 for a donation but the sudden demand for convalescent plasma from people who have recovered from COVID-19 commands a premium price, sometimes as high as $200. These donors are part of a fast-growing plasma industry that surpassed $25 billion in 2018. The U.S. supplies about three-quarters of the world's needs for plasma.
Payment for whole blood donation in the U.S. is prohibited, and while payment for plasma is allowed, there is a stigma attached to payment and much plasma is donated for free.
The pharmaceutical industry has shied away from natural products they cannot patent but they have identified simpler components from plasma, such as clotting factors and immunoglobulins, that have been turned into useful drugs from this raw material of plasma. While some companies have retooled to provide convalescent plasma to treat COVID-19, often paying those donors who have recovered a premium of several times the normal rate, most convalescent plasma has come as donations through traditional blood centers.
In April the Mayo Clinic, in cooperation with the FDA, created an expanded access program for convalescent plasma to treat COVID-19. It was meant to reduce the paperwork associated with gaining access to a treatment not yet approved by the FDA for that disease. Initially it was supposed to be for 5000 units but it quickly grew to more than twenty times that size. Michael Joyner, the head of the program, discussed that experience in an extended interview in September.
The Centers for Medicare and Medicaid Services (CMS) also created associated reimbursement codes, which became permanent in August.
Mayo published an analysis of the first 35,000 patients as a preprint in August. It concluded, "The relationships between mortality and both time to plasma transfusion, and antibody levels provide a signature that is consistent with efficacy for the use of convalescent plasma in the treatment of hospitalized COVID-19 patients."
It seemed to work best when given early in infection and in larger doses; a similar pattern has been seen in studies of monoclonal antibodies. A revised version will soon be published in a major medical journal. Some criticized the findings as not being from a randomized clinical trial.
Convalescent plasma is not the only intervention that seems to work better when used earlier in the course of disease. Recently the pharmaceutical company Eli Lilly stopped a clinical trial of a monoclonal antibody in hospitalized COVID-19 patients when it became apparent it wasn't helping. It is continuing trials for patients who are less sick and begin treatment earlier, as well as in persons who have been exposed to the virus but not yet diagnosed as infected, to see if it might prevent infection. In November the FDA eased access to this drug outside of clinical trials, though it is not yet approved for sale.
Show Me the Money
The antibodies that seem to give plasma its curative powers are fragile proteins that the body produces to fight the virus. Production shuts down once the virus is cleared and the remaining antibodies survive only for a few weeks before the levels fade. [Vaccines are used to train immune cells to produce antibodies and other defenses to respond to exposure to future pathogens.] So they can be usefully harvested from a recovered patient for only a few short weeks or months before they decline precipitously. The question becomes, how does one mobilize this resource in that short window of opportunity?
The program run by the Mayo Clinic explains the process and criteria for donating convalescent plasma for COVID-19, as well as links to local blood centers equipped to handle those free donations. Commercial plasma centers also are advertising and paying for donations.
A majority of countries prohibit paying donors for blood or blood products, including India. But an investigation by India Today touted a black market of people willing to donate convalescent plasma for the equivalent of several hundred dollars. Officials vowed to prosecute, saying donations should be selfless.
But that enforcement threat seemed to be undercut when the health minister of the state of Assam declared "plasma donors will get preference in several government schemes including the government job interview." It appeared to be a form of compensation that far surpassed simple cash.
The small city of Rexburg, Idaho, with a population a bit over 50,000, overwhelmingly Mormon and home to a campus of Brigham Young University, at one point had one of the highest per capita rates of COVID-19 in the current wave of infection. Rumors circulated that some students were intentionally trying to become infected so they could later sell their plasma for top dollar, potentially as much as $200 a visit.
Troubled university officials investigated the allegations but could come up with nothing definitive; how does one prove intentionality with such an omnipresent yet elusive virus? They chalked it up to idle chatter, perhaps an urban legend, which might be associated with alcohol use on some other campus.
Doctors, hospitals, and drug companies are all rightly praised for their altruism in the fight against COVID-19, but they also get paid. Payment for whole blood donation in the U.S. is prohibited, and while payment for plasma is allowed, there is a stigma attached to payment and much plasma is donated for free. "Why do we expect the donors [of convalescent plasma] to be the only uncompensated people in the process? It really makes no sense," argues Mark Yarborough, an ethicist at the UC Davis School of Medicine in Sacramento.
"When I was in grad school, two of my closest friends, at least once a week they went and gave plasma. That was their weekend spending money," Yarborough recalls. He says upper and middle-income people may have the luxury of donating blood products but prohibiting people from selling their plasma is a bit paternalistic and doesn't do anything to improve the economic status of poor people.
"Asking people to dedicate two hours a week for an entire year in exchange for cookies and milk is demonstrably asking too much," says Peter Jaworski, an ethicist who teaches at Georgetown University.
He notes that companies that pay plasma donors have much lower total costs than do operations that rely solely on uncompensated donations. The companies have to spend less to recruit and retain donors because they increase payments to encourage regular repeat donations. They are able to more rationally schedule visits to maximize use of expensive apheresis equipment and medical personnel used for the collection.
It seems that COVID-19 has been with us forever, but in reality it is less than a year. We have learned much over that short time, can now better manage the disease, and have lower mortality rates to prove it. Just how much convalescent plasma may have contributed to that remains an open question. Access to vaccines is months away for many people, and even then some people will continue to get sick. Given the lack of proven treatments, it makes sense to keep plasma as part of the mix, and not close the door to any legitimate means to obtain it.
Tapping into the Power of the Placebo Effect
When Wayne Jonas was in medical school 40 years ago, doctors would write out a prescription for placebos, spelling it out backwards in capital letters, O-B-E-C-A-L-P. The pharmacist would fill the prescription with a sugar pill, recalls Jonas, now director of integrative health programs at the Samueli Foundation. It fulfilled the patient's desire for the doctor to do something when perhaps no drug could help, and the sugar pills did no harm.
Today, that deception is seen as unethical. But time and time again, studies have shown that placebos can have real benefits. Now, researchers are trying to untangle the mysteries of placebo effect in an effort to better treat patients.
The use of placebos took off in the post-WWII period, when randomized controlled clinical trials became the gold standard for medical research. One group in a study would be treated with a placebo, a supposedly inert pill or procedure that would not affect normal healing and recovery, while another group in the study would receive an "active" component, most commonly a pill under investigation. Presumably, the group receiving the active treatment would have a better response and the difference from the placebo group would represent the efficacy of the drug being tested. That was the basis for drug approval by the U.S. Food and Drug Administration.
"Placebo responses were marginalized," says Ted Kaptchuk, director of the Program in Placebo Studies & Therapeutic Encounters at Harvard Medical School. "Doctors were taught they have to overcome it when they were thinking about using an effective drug."
But that began to change around the turn of the 21st century. The National Institutes of Health held a series of meetings to set a research agenda and fund studies to answer some basic questions, led by Jonas who was in charge of the office of alternative medicine at the time. "People spontaneously get better all the time," says Kaptchuk. The crucial question was, is the placebo effect real? Is it more than just spontaneous healing?
Brain mechanisms
A turning point came in 2001 in a paper in Science that showed physical evidence of the placebo effect. It used positron emission tomography (PET) scans to measure release patterns of dopamine — a chemical messenger involved in how we feel pleasure — in the brains of patients with Parkinson's disease. Surprisingly, the placebo activated the same patterns that were activated by Parkinson's drugs, such as levodopa. It proved the placebo effect was real; now the search was on to better understand and control it.
A key part of the effect can be the beliefs, expectations, context, and "rituals" of the encounter between doctor and patient. Belief by the doctor and patient that the treatment would work, and the formalized practices of administering the treatment can all contribute to a positive outcome.
Conditioning can be another important component in generating a response, as Pavlov demonstrated more than a century ago in his experiments with dogs. They were trained with a bell prior to feeding such that they would begin to salivate in anticipation at the sound of a bell even with no food present.
Translating that to humans, studies with pain medications and sleeping aids showed that patients who had a positive response with a certain dose of those medications could have the same response if the doses was reduced and a dummy pill substituted, even to the point where there was no longer any active ingredient.
Researchers think placebo treatments can work particularly well in helping people deal with pain and psychological disorders.
Those types of studies troubled Kaptchuk because they often relied on deception; patients weren't told they were receiving a placebo, or at best there was a possibility that they might be randomized to receive a placebo. He believed the placebo effect could work even if patients were told upfront that they were going to receive a placebo. More than a dozen so call "open-label placebo" studies across numerous medical conditions, by Kaptchuk and others, have shown that you don't have to lie to patients for a placebo to work.
Jonas likes to tell the story of a patient who used methotrexate, a potent immunosuppressant, to control her rheumatoid arthritis. She was planning a long trip and didn't want to be bothered with the injections and monitoring required in using the drug, So she began to drink a powerful herbal extract of anise, a licorice flavor that she hated, prior to each injection. She reduced the amount of methotrexate over a period of months and finally stopped, but continued to drink the anise. That process had conditioned her body "to alter her immune function and her autoimmunity" as if she were taking the drug, much like Pavlov's dogs had been trained. She has not taken methotrexate for more than a year.
An intriguing paper published in May 2021 found that mild, non-invasive electric stimulation to the brain could not only boost the placebo effect on pain but also reduce the "nocebo" effect — when patients report a negative effect to a sham treatment. While the work is very preliminary, it may open the door to directly manipulating these responses.
Researchers think placebo treatments can work particularly well in helping people deal with pain and psychological disorders, areas where drugs often are of little help. Still, placebos aren't a cure and only a portion of patients experience a placebo effect.
Nocebo
If medicine were a soap opera, the nocebo would be the evil twin of the placebo. It's what happens when patients have adverse side effects because of the expectation that they will. It's commonly seem when patients claims to experience pain or gastric distress that can occur with a drug even when they've received a placebo. The side effects were either imagined or caused by something else.
"Up to 97% of reported pharmaceutical side effects are not caused by the drug itself but rather by nocebo effects and symptom misattribution," according to one 2019 paper.
One way to reduce a nocebo response is to simply not tell patients that specific side effects might occur. An example is a liver biopsy, in which a large-gauge needle is used to extract a tissue sample for examination. Those told ahead of time that they might experience some pain were more likely to report pain and greater pain than those who weren't offered this information.
Interestingly, a nocebo response plays out in the hippocampus, a part of the brain that is never activated in a placebo response. "I think what we are dealing with with nocebo is anxiety," says Kaptchuk, but he acknowledges that others disagree.
Distraction may be another way to minimize the nocebo effect. Pediatricians are using virtual reality (VR) to engage children and distract them during routine procedures such as blood draws and changing wound dressings, and burn patients of all ages have found relief with specially created VRs.
Treatment response
Jonas argues that what we commonly call the placebo effect is misnamed and leading us astray. "The fact is people heal and that inherent healing capacity is both powerful and influenced by mental, social, and contextual factors that are embedded in every medical encounter since the idea of treatment began," he wrote in a 2019 article in the journal Frontiers in Psychiatry. "Our understanding of healing and ability to enhance it will be accelerated if we stop using the term 'placebo response' and call it what it is—the meaning response, and its special application in medicine called the healing response."
He cites evidence that "only 15% to 20% of the healing of an individual or a population comes from health care. The rest—nearly 80%—comes from other factors rarely addressed in the health care system: behavioral and lifestyle choices that people make in their daily life."
To better align treatments and maximize their effectiveness, Jonas has created HOPE (Healing Oriented Practices & Environments) Note, "a patient-guided process designed to identify the patient's values and goals in their life and for healing." Essentially, it seeks to make clear to both doctor and patient what the patient's goals are in seeking treatment. In an extreme example of terminal cancer, some patients may choose to extend life despite the often brutal treatments, while others might prefer to optimize quality of life in the remaining time that they have. It builds on practices already taught in medical schools. Jonas believes doctors and patients can use tools like these to maximize the treatment response and achieve better outcomes.
Much of the medical profession has been resistant to these approaches. Part of that is simply tradition and limited data on their effectiveness, but another very real factor is the billing process for how they are reimbursed. Jonas says a new medical billing code added this year gives doctors another way to be compensated for the extra time and effort that a more holistic approach to medicine may initially require. Other moves away from fee-for-service payments to bundling and payment for outcomes, and the integrated care provided by the Veterans Affairs, Kaiser Permanente and other groups offer longer term hope for the future of approaches that might enhance the healing response.
This article was first published by Leaps.org on July 7, 2021.
New tech helps people of all ages stay social
Sonja Bauman, 39, and Mariela Florez, 83, taking one of their regular walks in Plantation, Fla. The pair met through an online platform called Papa that provides "family on demand." (Photo by Sonja Bauman)
In March, Sonja Bauman, 39, used an online platform called Papa, which offers “family on demand,” to meet Mariela Florez, an 83-year-old retiree. Despite living with her adult children, Florez was bored and lonely when they left for work, and her recoveries from a stroke and broken hip were going slowly. That's when Bauman began visiting twice per week. They take walks, strengthening Florez’s hip, and play games like Connect Four for mental stimulation. “It’s very important for me so I don’t feel lonely all day long,” said Florez. Her memories, blurred by the stroke, are gradually returning.
Papa is one of a growing number of tech approaches that are bringing together people of all ages. In addition to platforms like Papa that connect people in real life, other startups use virtual reality and video, with some of them focusing especially on deepening social connections between the generations — relationships that support the health of older and younger people alike. “I enjoy seeing Mariela as much as she enjoys seeing me,” Bauman said.
Connecting in real life
Telehealth expert Andrew Parker founded Papa in 2017 to improve the health outcomes of older adults and families. Seniors can meet people — some their grandkids’ age — for healthy activities, while working parents find retirees to watch their children. These “Papa Pals” are provided as a benefit through Medicare, Medicaid and some employer health plans.
In 2020, Papa connected Bauman, the 39-year-old Floridian, with another woman in her mid-70s who lives alone and has very limited mobility. Bauman began driving her to doctor’s appointments and helping her with chores around the house. “When I’m not there, she doesn’t leave her apartment,” said Bauman. The two have gone to the gym together, and they walk slowly through the neighborhood, chatting so it feels less like exercise.
Parker was driven to start Papa by the problem of social isolation among seniors, exacerbated by the pandemic, but he believes users of all ages can benefit. “Many of our Pals feel more comfortable opening up with older members than their same-aged friends,” he said.
Other platforms aim for similar, in-person connections. Generation Tech unites teens with seniors for technology training. And Mon Ami, which provides case management software for aging and disability service providers, has an app that connects isolated older people with college-age volunteers.
Making new connections through video
Several new sites match you with strangers for real-time video chatting on various topics, such as finding common ground on political issues. Other video platforms focus on intergenerational connections.
S. Jay Olshansky, a gerontology professor at the University of Illinois-Chicago, recalls the first time he saw Hyunseung Lee, an 11-year-old from Seoul, through his computer screen. The kid was shy, but Olshansky, 67, encouraged him to ask questions. “Turns out, he was thirsting for this kind of interaction.”
They’d connected through Eldera, a platform that pairs mentors age 60 and up with mentees, using an algorithm, for video conversations. “The time and wisdom of older adults is the most important natural resource we can give future generations,” said Dana Griffin, Eldera's CEO. “Connecting through a screen is the opposite of social media.”
In weekly meetings, Olshansky noticed Lee’s unique interest in math. “There’s something special in you,” Olshansky told him. “How do we bring it to the surface?” He suggested Lee write a book on his favorite subject, and the preteen ran with it, cranking out 70 pages in two weeks. Lee has published his love letter to theorems on Amazon.
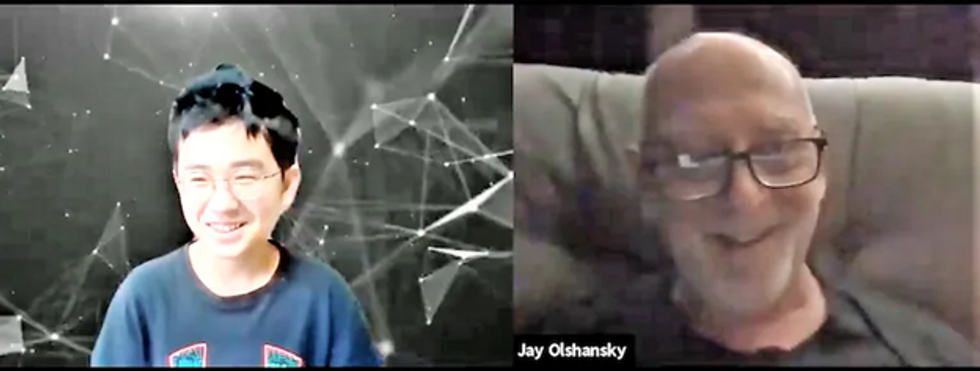
Hyunseung Lee, age 11, of Korea, and U.S. college professor Jay Olshanksy, 67, discuss math, strategy and Hyunsung's budding career as a book author during their video chats through a platform called Eldera. (Photo by Dana Palmer/Eldera)
Lee’s parents told Olshansky that their son has become more assertive — a recurring theme, Griffin said. “Confidence is the number one thing parents tell us about.” Since Eldera’s inception last year, the number of mentors has grown exponentially. Even so, Griffin said the waitlist for mentors typically numbers 200 kids.
Another site, Big and Mini, hosts video interactions between seniors and young adults; about 10,000 active users have joined since 2019, said co-founder Aditi Merchant.
Users often bring the benefits of their video interactions to their real-world relationships. Olshansky views Lee as an older version of his grandkids. “Eldera teaches me how to interact with them.” Lee, high on confidence, began instructing his classmates in math. Griffin noted that a group of Eldera mentors in Memphis, who met initially on Eldera, now take walks together in-person to trade ideas for helping each other’s Eldera kids solve problems in their schools and communities.
“We’ve evolved into a community for older adults who want to give back to the world,” said Griffin. Other new tools for connection take the form of virtual reality apps.
Connecting in virtual reality
During pandemic isolation, record numbers of people bought devices for virtual and augmented reality. Such gadgets can convince you that you’re hanging out with friends, even if they’re in another hemisphere. Lifelike simulations from miles away could be especially useful for meaningful interactions between people of different generations, since they’re often geographically segregated.
VR’s benefits require further study, but users report less social isolation and depression, according to MIT research. The immersive, 3-D experience is more compelling than FaceTime or Zoom. “It’s like the difference between a phone call and video call,” said Rick Robinson, Vice President of AARP’s Innovation Labs.
“When VR is designed right, the medium disappears,” said Jeremy Bailenson of Stanford.
Dana Pierce, a 56-year-old government employee in Indiana, got Meta's VR headset in May, 2021, thinking she’d enjoy it more than a new laptop. After many virtual group tours of exotic destinations, she has no regrets. Her adventures occur on Alcove, an app made by Robinson’s Innovation Labs. He co-created it with VR-company Rendever and sought input from people over age 50 to tailor it to their interests. “I’m an introvert,” said Pierce. “I’ve been more socially active since getting my headset than I am in real life.”
Tagging along with her to places like Paris are avatars representing real people around the world. She’s gotten to know VR users in their 70s, 80s and 90s, as well as younger people and some her own age. One is a new friend she plays chess with in relaxing nature settings. Another is her oldest son. He lives 90 minutes away but, earlier this year, Pierce welcomed him and his girlfriend to her virtual house on Alcove. They chatted in the living room decorated with family photos uploaded by Pierce. Then they took out a boat to go VR fishing — because why not — until 2 a.m.
“When VR is designed right, the medium disappears,” said Jeremy Bailenson, a communications professor who directs Stanford’s Virtual Human Interaction Lab. He’s teaching a class of 175 students entirely in VR. After months of covid isolation, the first time the class met, “there was a big catharsis. It really feels like you’re in a big crowd.” Like-minded people meet in VR for events such as comedy shows and creative writing meetups, while the Swedish pop group ABBA has performed this year as digital versions of themselves (“ABBA-tars”) during a virtual concert tour.
Karen Fingerman, a psychologist and director of the Texas Aging and Longevity Center at the University of Texas-Austin, supports the idea of VR for social connection, though she added that some people need it more than others. Hospitals and assisted-living facilities are using products such as Penumbra’s REAL I-Series and MyndVR to bring VR excursions to isolated patients and seniors. “If you’re in a bed or facility, this gives you something to talk about,” said Gita Barry, Penumbra’s executive vice president.
Pierce uses it on most days. She may see another adult son, who lives with her, less often as a result. But VR helps her manage real-world stressors, more than escaping them. After a long workday, she visits her back porch on Alcove, which overlooks a pond. “It’s my little retreat,” she said. “VR improves my mood. It’s added a lot to my life.”
Some seniors are using more than one technology. Olshansky and Lee discuss strategy while playing Internet chess. And Olshansky recently began using VR. He sees his sister, who lives far away, in a virtual beach house. “It’d be a great way to interact with Hyunseung,” he said. “I should get him a headset.”
A version of this article first appeared in The Washington Post on December 3, 2021.

