A New Stem Cell Therapy Provides Hope to Patients with Blood Cancer
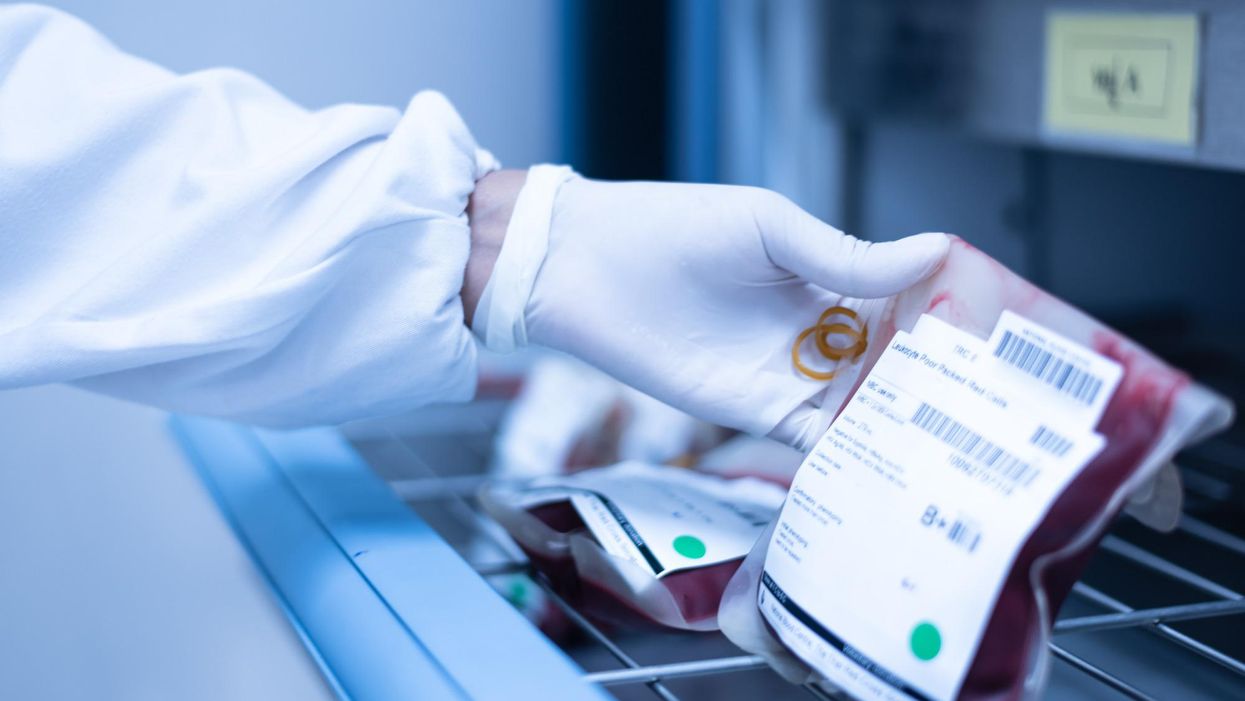
Stacey Khoury felt more fatigued and out of breath than she was used to from just walking up the steps to her job in retail jewelry sales in Nashville, Tennessee. By the time she got home, she was more exhausted than usual, too.
"I just thought I was working too hard and needed more exercise," recalls the native Nashvillian about those days in December 2010. "All of the usual excuses you make when you're not feeling 100%."
As a professional gemologist, being hospitalized during peak holiday sales season wasn't particularly convenient. There was no way around it though when her primary care physician advised Khoury to see a blood disorder oncologist because of her disturbing blood count numbers. As part of a routine medical exam, a complete blood count screens for a variety of diseases and conditions that affect blood cells, such as anemia, infection, inflammation, bleeding disorders and cancer.
"If approved, it will allow more patients to potentially receive a transplant than would have gotten one before."
While she was in the hospital, a bone marrow biopsy revealed that Khoury had acute myeloid leukemia, or AML, a high-risk blood cancer. After Khoury completed an intense first round of chemotherapy, her oncologist recommended a bone marrow transplant. The potentially curative treatment for blood-cancer patients requires them to first receive a high dose of chemotherapy. Next, an infusion of stem cells from a healthy donor's bone marrow helps form new blood cells to fight off the cancer long-term.
Each year, approximately 8,000 patients in the U.S. with AML and other blood cancers receive a bone marrow transplant from a donor, according to the Center for International Blood and Marrow Transplant Research. But Khoury wasn't so lucky. She ended up being among the estimated 40% of patients eligible for bone marrow transplants who don't receive one, usually because there's no matched donor available.
Khoury's oncologist told her about another option. She could enter a clinical trial for an investigational cell therapy called omidubicel, which is being developed by Israeli biotech company Gamida Cell. The company's cell therapy, which is still experimental, could up a new avenue of treatment for cancer patients who can't get a bone marrow transplant.
Omidubicel consists of stem cells from cord blood that have been expanded using Gamida's technology to ensure there are enough cells for a therapeutic dose. The company's technology allows the immature cord blood cells to multiply quickly in the lab. Like a bone marrow transplant, the goal of the therapy is to make sure the donor cells make their way to the bone marrow and begin producing healthy new cells — a process called engraftment.
"If approved, it will allow more patients to potentially receive a transplant than would have gotten one before, so there's something very novel and exciting about that," says Ronit Simantov, Gamida Cell's chief medical officer.
Khoury and her husband Rick packed up their car and headed to the closest trial site, the Duke University School of Medicine, roughly 500 miles away. There they met with Mitchell Horowitz, a stem cell transplant specialist at Duke and principal investigator for Gamida's omidubicel study in the U.S.
He told Khoury she was a perfect candidate for the trial, and she enrolled immediately. "When you have one of two decisions, and it's either do this or you're probably not going to be around, it was a pretty easy decision to make, and I am truly thankful for that," she says.
Khoury's treatment started at the end of March 2011, and she was home by July 4 that year. She say the therapy "worked the way the doctors wanted it to work." Khoury's blood counts were rising quicker than the people who had bone marrow matches, and she was discharged from Duke earlier than other patients were.
By expanding the number of cord blood cells — which are typically too few to treat an adult — omidubicel allows doctors to use cord blood for patients who require a transplant but don't have a donor match for bone marrow.
Patients receiving omidubicel first get a blood test to determine their human leukocyte antigen, or HLA, type. This protein is found on most cells in the body and is an important regulator of the immune system. HLA typing is used to match patients to bone marrow and cord blood donors, but cord blood doesn't require as close of a match.
Like bone marrow transplants, one potential complication of omidubicel is graft-versus-host disease, when the donated bone marrow or stem cells register the recipient's body as foreign and attack the body. Depending on the severity of the response, according to the Mayo Clinic, treatment includes medication to suppress the immune system, such as steroids. In clinical trials, the occurrence of graft-versus-host disease with omidubicel was comparable with traditional bone marrow transplants.
"Transplant doctors are working on improving that," Simantov says. "A number of new therapies that specifically address graft-versus-host disease will be making some headway in the coming months and years."
Gamida released the results of the Phase 3 study in February and continues to follow Khoury and the other study patients for their long-term outcomes. The large randomized trial evaluated the safety and efficacy of omidubicel compared to standard umbilical cord blood transplants in patients with blood cancer who didn't have a suitable bone marrow donor. Around 120 patients aged 12 to 65 across the U.S., Europe and Asia were included in the trial. The study found that omidubicel resulted in faster recovery, fewer bacterial and viral infections and fewer days in the hospital.
The company plans to seek FDA approval this year. Simantov anticipates the therapy will receive FDA approval by 2022.
"Opening up cord blood transplants is very important, especially for people of diverse ethnic backgrounds," says oncologist Gary Schiller, principal investigator at the David Geffen School of Medicine at UCLA for Gamida Cell's mid- and late-stage trials. "This expansion technology makes a big difference because it makes cord blood an available option for those who do not have another donor source."
As for Khoury, who proudly celebrated the anniversary of her first transplant in April—she remains cancer free and continues to work full-time as a gemologist. When she has a little free time, she enjoys gardening, sewing, or maybe traveling to national parks like Yellowstone or the Grand Canyon with her husband Rick.
A new type of cancer therapy is shrinking deadly brain tumors with just one treatment
MRI scans after a new kind of immunotherapy for brain cancer show remarkable progress in one patient just days after the first treatment.
Few cancers are deadlier than glioblastomas—aggressive and lethal tumors that originate in the brain or spinal cord. Five years after diagnosis, less than five percent of glioblastoma patients are still alive—and more often, glioblastoma patients live just 14 months on average after receiving a diagnosis.
But an ongoing clinical trial at Mass General Cancer Center is giving new hope to glioblastoma patients and their families. The trial, called INCIPIENT, is meant to evaluate the effects of a special type of immune cell, called CAR-T cells, on patients with recurrent glioblastoma.
How CAR-T cell therapy works
CAR-T cell therapy is a type of cancer treatment called immunotherapy, where doctors modify a patient’s own immune system specifically to find and destroy cancer cells. In CAR-T cell therapy, doctors extract the patient’s T-cells, which are immune system cells that help fight off disease—particularly cancer. These T-cells are harvested from the patient and then genetically modified in a lab to produce proteins on their surface called chimeric antigen receptors (thus becoming CAR-T cells), which makes them able to bind to a specific protein on the patient’s cancer cells. Once modified, these CAR-T cells are grown in the lab for several weeks so that they can multiply into an army of millions. When enough cells have been grown, these super-charged T-cells are infused back into the patient where they can then seek out cancer cells, bind to them, and destroy them. CAR-T cell therapies have been approved by the US Food and Drug Administration (FDA) to treat certain types of lymphomas and leukemias, as well as multiple myeloma, but haven’t been approved to treat glioblastomas—yet.
CAR-T cell therapies don’t always work against solid tumors, such as glioblastomas. Because solid tumors contain different kinds of cancer cells, some cells can evade the immune system’s detection even after CAR-T cell therapy, according to a press release from Massachusetts General Hospital. For the INCIPIENT trial, researchers modified the CAR-T cells even further in hopes of making them more effective against solid tumors. These second-generation CAR-T cells (called CARv3-TEAM-E T cells) contain special antibodies that attack EFGR, a protein expressed in the majority of glioblastoma tumors. Unlike other CAR-T cell therapies, these particular CAR-T cells were designed to be directly injected into the patient’s brain.
The INCIPIENT trial results
The INCIPIENT trial involved three patients who were enrolled in the study between March and July 2023. All three patients—a 72-year-old man, a 74-year-old man, and a 57-year-old woman—were treated with chemo and radiation and enrolled in the trial with CAR-T cells after their glioblastoma tumors came back.
The results, which were published earlier this year in the New England Journal of Medicine (NEJM), were called “rapid” and “dramatic” by doctors involved in the trial. After just a single infusion of the CAR-T cells, each patient experienced a significant reduction in their tumor sizes. Just two days after receiving the infusion, the glioblastoma tumor of the 72-year-old man decreased by nearly twenty percent. Just two months later the tumor had shrunk by an astonishing 60 percent, and the change was maintained for more than six months. The most dramatic result was in the 57-year-old female patient, whose tumor shrank nearly completely after just one infusion of the CAR-T cells.
The results of the INCIPIENT trial were unexpected and astonishing—but unfortunately, they were also temporary. For all three patients, the tumors eventually began to grow back regardless of the CAR-T cell infusions. According to the press release from MGH, the medical team is now considering treating each patient with multiple infusions or prefacing each treatment with chemotherapy to prolong the response.
While there is still “more to do,” says co-author of the study neuro-oncologist Dr. Elizabeth Gerstner, the results are still promising. If nothing else, these second-generation CAR-T cell infusions may someday be able to give patients more time than traditional treatments would allow.
“These results are exciting but they are also just the beginning,” says Dr. Marcela Maus, a doctor and professor of medicine at Mass General who was involved in the clinical trial. “They tell us that we are on the right track in pursuing a therapy that has the potential to change the outlook for this intractable disease.”
A recent study in The Lancet Oncology showed that AI found 20 percent more cancers on mammogram screens than radiologists alone.
Since the early 2000s, AI systems have eliminated more than 1.7 million jobs, and that number will only increase as AI improves. Some research estimates that by 2025, AI will eliminate more than 85 million jobs.
But for all the talk about job security, AI is also proving to be a powerful tool in healthcare—specifically, cancer detection. One recently published study has shown that, remarkably, artificial intelligence was able to detect 20 percent more cancers in imaging scans than radiologists alone.
Published in The Lancet Oncology, the study analyzed the scans of 80,000 Swedish women with a moderate hereditary risk of breast cancer who had undergone a mammogram between April 2021 and July 2022. Half of these scans were read by AI and then a radiologist to double-check the findings. The second group of scans was read by two researchers without the help of AI. (Currently, the standard of care across Europe is to have two radiologists analyze a scan before diagnosing a patient with breast cancer.)
The study showed that the AI group detected cancer in 6 out of every 1,000 scans, while the radiologists detected cancer in 5 per 1,000 scans. In other words, AI found 20 percent more cancers than the highly-trained radiologists.

But even though the AI was better able to pinpoint cancer on an image, it doesn’t mean radiologists will soon be out of a job. Dr. Laura Heacock, a breast radiologist at NYU, said in an interview with CNN that radiologists do much more than simply screening mammograms, and that even well-trained technology can make errors. “These tools work best when paired with highly-trained radiologists who make the final call on your mammogram. Think of it as a tool like a stethoscope for a cardiologist.”
AI is still an emerging technology, but more and more doctors are using them to detect different cancers. For example, researchers at MIT have developed a program called MIRAI, which looks at patterns in patient mammograms across a series of scans and uses an algorithm to model a patient's risk of developing breast cancer over time. The program was "trained" with more than 200,000 breast imaging scans from Massachusetts General Hospital and has been tested on over 100,000 women in different hospitals across the world. According to MIT, MIRAI "has been shown to be more accurate in predicting the risk for developing breast cancer in the short term (over a 3-year period) compared to traditional tools." It has also been able to detect breast cancer up to five years before a patient receives a diagnosis.
The challenges for cancer-detecting AI tools now is not just accuracy. AI tools are also being challenged to perform consistently well across different ages, races, and breast density profiles, particularly given the increased risks that different women face. For example, Black women are 42 percent more likely than white women to die from breast cancer, despite having nearly the same rates of breast cancer as white women. Recently, an FDA-approved AI device for screening breast cancer has come under fire for wrongly detecting cancer in Black patients significantly more often than white patients.
As AI technology improves, radiologists will be able to accurately scan a more diverse set of patients at a larger volume than ever before, potentially saving more lives than ever.

