Six Questions about the Kids' COVID Vaccine, Answered by an Infectious Disease Doctor

The author, an infectious disease physician, pictured with his two daughters who are getting vaccinated against COVID-19.
I enthusiastically support the vaccination against COVID for children aged 5-11 years old. As an infectious disease doctor who took care of hundreds of COVID-19 patients over the past 20 months, I have seen the immediate and long-term consequences of COVID-19 on patients – and on their families. As a father of two daughters, I have lived through the fear and anxiety of protecting my kids at all cost from the scourges of the pandemic and worried constantly about bringing the virus home from work.
It is imperative that we vaccinate as many children in the community as possible. There are several reasons why. First children do get sick from COVID-19. Over the course of the pandemic in the U.S, more than 2 million children aged 5-11 have become infected, more than 8000 have been hospitalized, and more than 100 have died, making COVID one of the top 10 causes of pediatric deaths in this age group over the past year. Children are also susceptible to chronic consequences of COVID such as long COVID and multisystem inflammatory syndrome in children (MIS-C). Most studies demonstrate that 10-30% of children will develop chronic symptoms following COVID-19. These include complaints of brain fog, fatigue, trouble breathing, fever, headache, muscle and joint pains, abdominal pain, mood swings and even psychiatric disorders. Symptoms typically last from 4-8 weeks in children, with some reporting symptoms that persist for many months.
Second, children are increasingly recognized as vectors who can bring infection into the house, potentially transmitting infection to vulnerable household members. Finally, we have all seen the mayhem that results when one child in the classroom becomes infected with COVID and the other students get sent home to quarantine – across the U.S., more than 2000 schools have been affected this way.
We now have an extraordinarily effective vaccine with more than 90 percent efficacy at preventing symptomatic infection. Vaccinating children will boost our countrywide vaccination rate which is trailing many countries after an early start. Nevertheless, there are still many questions and concerns that parents have as the vaccine gets rolled out. I will address six of them here.
"Novel Vaccine Technology"
Even though this is a relatively new vaccine, the technology is not new. Scientists had worked on mRNA vaccines for decades prior to the COVID mRNA vaccine breakthrough. Furthermore, experience with the Pfizer COVID vaccine is rapidly growing. By now it has been more than a year and a half since the Pfizer trials began in March 2020, and more than 7 billion doses have already been administered globally, including in 13.7 million adolescents in the U.S. alone.
"Will This Vaccine Alter My Child's DNA?"
No. This is not how mRNA works. DNA is present in the cell's nucleus. The mRNA only stays in the outside cytoplasm, gets destroyed and never enters the inner sanctum of the nucleus. Furthermore, for the mRNA to be ever integrated into DNA, it requires a special enzyme called reverse transcriptase which humans don't have. Proteins (that look like the spike proteins on SARS-CoV-2) are made directly from this mRNA message without involvement of our DNA at any time. Pieces of spike proteins get displayed on the outside of our cells and our body makes protective antibodies that then protects us handily against the future real virus if it were ever to enter our (or our children's) bodies. Our children's DNA or genes can never be affected by an mRNA vaccine.
"Lack of Info on Long-Term Side Effects"
Unlike medications that are taken daily or periodically and can build up over time, the mRNA in the Pfizer vaccine is evanescent. It literally is just the messenger (that is what the "m" in mRNA stands for) and the messenger quickly disappears. mRNA is extremely fragile and easily inactivated – that's why we need to encase it in a special fatty bubble and store the vaccines at extremely cold temperatures. Our cells break down and destroy the mRNA within a few days after receiving the instructions to make the virus spike proteins. The presence of these fragments of the virus (note this is not "live" virus) prompts our immune system to generate protective antibodies to the real thing. Our bodies break down mRNA all the time in normal cellular processes – this is nothing new.
What the transience of the delivery system means is that most of the effects of the mRNA vaccines are expected to be more immediate (sore arm, redness at the site, fever, chills etc.), with no long-term side effects anticipated. A severe allergic response has been reported to occur in some generally within the first 15 minutes, is very rare, and everyone gets observed for that as part of standard vaccine administration. Even with the very uncommon complication of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining of the heart) seen primarily in young men under the age of 30 following mRNA vaccines, these typically happen within days to 2 weeks and many return to work or school in days. In the 70-year history of pediatric (and adult vaccines), dangerous complications happen in the first two months. There have been millions of adolescents as young as 12 years and thousands in the initial trial of children aged 5-11 who have already received the vaccine and are well beyond the two-month period of observation. There is no biological reason to believe that younger children will have a different long-term side effect profile compared to adolescents or adults.
"Small Sample Size in Kids and the Trial Design"
Although the Pfizer trial in children aged 5-11 was relatively small, it was big enough to give us statistical confidence in assessing safety and efficacy outcomes. Scientists spend a lot of time determining the right sample size of a study during the design phase. On one hand, you want to conduct the study efficiently so that resources are used in a cost-effective way and that you get a timely answer, especially in a fast-moving pandemic. On the other hand, you want to make sure you have enough sample size so that you can answer the question confidently as to whether the intervention works and whether there are adverse effects. The more profound the effect size of the intervention (in this case the vaccine), the fewer the numbers of children needed in the trials.
Statistics help investigators determine whether the results seen would have appeared by chance or not. In this case, the effect was real and impressive. Over 3,000 children around the world have received the vaccines through the trials alone with no serious side effects detected. The first press release reported that the immune response in children aged 5-11 was similar (at one-third the vaccine dose) to the response in the comparator group aged 16-25 years old. Extrapolating clinical efficacy results from immune response measurements ("immunobridging" study) would already have been acceptable if this was the only data. This is a standard trial design for many pediatric vaccines. Vaccines are first tested in the lab, followed by animals then adults. Only when deemed safe in adults and various regulatory bodies have signed off, do the pediatric vaccine trials commence.
Because children's immune systems and bodies are in a constant state of development, the vaccines must be right-sized. Investigators typically conduct "age de-escalation" studies in various age groups. The lowest dose is first tried so see if that is effective, then the dose is increased gradually as needed. Immune response is the easiest, safest and most efficient way to test the efficacy of pediatric vaccines. This is a typical size and design of a childhood vaccine seeking regulatory approval. There is no reason to think that the clinical efficacy would be any different in children vs. adults for a given antibody response, given the experience already in the remainder of the population, including older children and adolescents. Although this was primarily designed as an "immunobridging" study, the initial immunologic response data was followed by real clinical outcomes in this population. Reporting on the outcomes of 2,268 children in the randomized controlled trial, the vaccine was 90.7% effective at preventing symptomatic infection.
"Fear of Myocarditis"
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining of the heart) have been associated with receipt of the mRNA vaccines, particularly among male adolescents and young adults, typically within a few days after receiving the second dose. But this is very rare. For every million vaccine recipients, you would expect 41 cases in males, and 4 cases in females aged 12-29 years-old. The risk in older age groups is substantially lower. It is important to recognize that the risk of myocarditis associated with COVID is substantially higher. Patients present with new chest pain, shortness of breath, or palpitations after receiving an mRNA vaccine (more common after the second dose). But outcomes are good if associated with the vaccine. Most respond well to treatment and resolve symptoms within a week. There have been no deaths associated with vaccine-associated myocarditis.
In contrast, COVID-associated myocarditis has been associated with more severe cases as well as other complications including chronic symptoms of long COVID. The risk of myocarditis is likely related to vaccine dose, so the fact that one-third the dose of the vaccine will be used in the 5-11 year-olds is expected to correspond to a lower risk of myocarditis. At the lower dose given to younger kids, there has been a lower incidence of adverse effects reported compared to older children and adults who received the full dose. In addition, baseline rates of myocarditis not associated with vaccination are much lower in children ages 5-11 years than in older children, so the same may hold true for vaccine-associated myocarditis cases. This is because myocarditis is associated with sex hormones (particularly testosterone) that surge during puberty. In support of this, the incidence of vaccine-associated myocarditis is lower in 12–15-year-old boys, compared to those who were older than 16 years old. There were no cases of myocarditis reported in the experience to date of 5–11-year-old children in the trials, although the trial was too small to pick up on such a rare effect.
"Optimal Dose Spacing Interval: Longer Than 3 Weeks?"
There is a biologic basis for increasing the interval between vaccine doses in general. Priming the immune system with the first shot and then waiting gives the second shot a better chance of prompting a secondary immune reaction that results in a more durable response (with more T cell driven immune memory). One study from the U.K. showed that the antibody response in people over 80 was more than 3 times higher if they delayed the second dose to after 12 weeks for the Pfizer vaccine instead of the 3 weeks studied in trials. In a study of 503 British health care workers, there were twice as many neutralizing antibodies produced in a longer interval group (6-14 weeks) versus a shorter interval group (3-4 weeks) between doses. However, the safety and efficacy with longer intervals has not been evaluated in the pediatric or other COVID vaccine trials.
In the U.S., the C.D.C. reported that 88 percent of counties are at a "high" or "substantial" level of community transmission. Also, Europe is already experiencing a winter surge of infections that may predict more U.S. winter cases as international travel reopens. During a time of high community virus burden with a highly transmissible Delta variant, relying on one dose of vaccine for several more weeks until the second may leave many more susceptible to infection while waiting. One study from England showed that one dose of the Pfizer vaccine was only 33% protective against symptomatic Delta infection in contrast to 50% for the Alpha variant in adults. There has been no corollary information in children but we would expect less protection in general from one vaccine dose vs. two. This is a particularly important issue with the upcoming holiday season when an increased number of families will travel. Some countries such as the U.K. and Norway have proceeded with only offering older than 12 year-olds one dose of vaccine rather than two, but this was before the current European surge which may change the risk-benefit calculus. There are no plans to only offer one vaccine dose in the U.S. at this time. However a lower dose of the vaccine will likely be studied in the future for adolescents aged 12-15.
For parents worried about the potential risk of adverse effects of two doses of vaccines in their children, it is reasonable to wait 6-12 weeks for the second shot but it all depends on your risk-benefit calculus. There is biological plausibility to pursue this strategy. Although there is no pediatric-specific data to draw from, a longer interval may lengthen immune memory and potentially decrease the risk of myocarditis, particularly in boys. There may only be partial benefit in eliciting protective antibodies after one vaccine dose but only 2-4% of children are hospitalized with COVID once infected, with risk of severe illness increasing if they have comorbidities.
There are also some data indicating that 40% of children have already been exposed to infection naturally and may not need further protection after one shot. However, this percentage is likely a large overestimation given the way the data was collected. Using antibody tests to ascertain previous infection in children may be problematic for several reasons: uncertainty regarding duration of protection, variability in symptoms in children with most having very mild symptoms, and the lack of standardization of antibody tests in general. Overall, if the child has medical comorbidities such as diabetes, parents are planning to travel with their children, if local epidemiology shows increasing cases, and if there are elderly or immunocompromised individuals in the household, I would vaccinate children with two doses as per the original recommended schedule.
Bottom line: Given the time of the year and circulating Delta, I would probably stick with the recommended 3-week interval between doses for now for most children. But if parents choose a longer interval between the first and second dose for their children, I wouldn't worry too much about it. Better to be vaccinated - even if slowly, over time -- than not at all.
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained
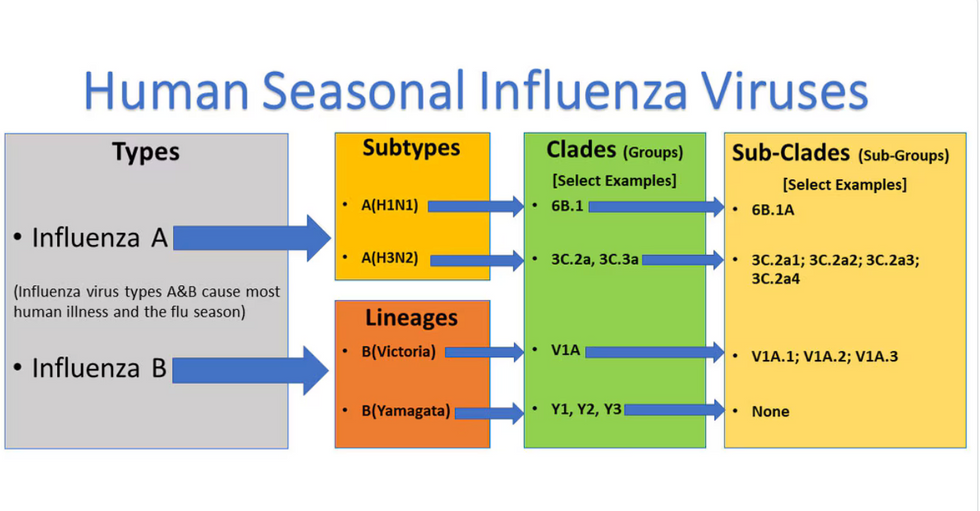
Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

