Deep Brain Stimulation for Mental Illnesses Raises Ethical Concerns
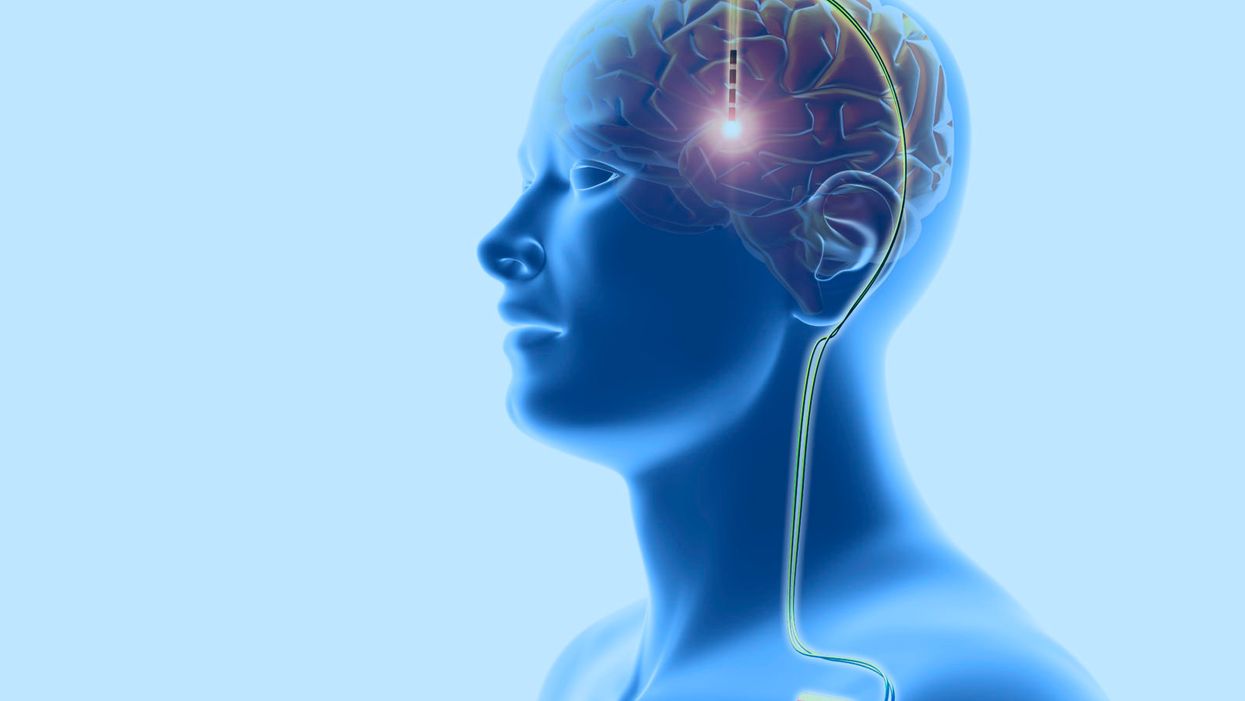
Deep brain stimulation: This neurosurgical treatment involves the implantation of electrodes in the cerebral lobes of the brain, linked through the scalp (top) to wires (down right) leading to a battery implanted below the skin. This sends electrical impulses to specific areas of the brain. DBS was developed for the treatment of Parkinson's disease, but is being investigated for use in other conditions.
Imagine that you are one of the hundreds of millions of people who suffer from depression. Medication hasn't helped you, so you're looking for another treatment option. Something powerful enough to change your mood as soon as you need a lift.
"If a participant experiences a personality change, does this change who they are or dehumanize them by altering their nature?"
Enter deep brain stimulation: a type of therapy in which one or more electrodes are inserted into your brain and connected to a surgically implanted, battery-operated medical device in your chest. This device, which is approximately the size of a stopwatch, sends electric pulses to a targeted region of your brain. The idea is to control a variety of neurological symptoms that can't be adequately managed by drugs.
Over the last twenty years, deep brain stimulation, known as DBS, has become an efficient and safe alternative for the treatment of chronic neurological diseases such as epilepsy, Parkinson's disease and neuropathic pain. According to the International Neuromodulation Society, there have been more than 80,000 deep brain stimulation implants performed around the world.
The Food and Drug Administration approved DBS as a treatment for essential tremor and Parkinson's in 1997, dystonia in 2003 and obsessive compulsive disorder in 2009. Since doctors can use drugs and treatments "off-label" (not approved by the FDA) to treat patients with any disease, DBS is now also being investigated as a treatment for chronic pain, PTSD and major depression.
And these new applications are raising profound ethical questions about individuality, personality, and even what it means to be human.
"These patients are essentially having a computer that can modify and influence emotional processing, mood and motor outputs inserted into the brain," said Gabriel Lazaro-Munoz, an assistant professor at The Center for Medical Ethics and Health Policy at Baylor College of Medicine. "These responses define us as human beings and dictate our autonomy. If a participant experiences a personality change, does this change who they are or dehumanize them by altering their nature? These are some of the questions we have to consider."
"When we are not in control of ourselves, are we ourselves?"
The U.S. government has similar concerns about DBS. The National Institutes of Health recently awarded grants to study the neuroethical issues surrounding the use of DBS in neuropsychiatric and movement disorders and appropriate consent for brain research. The grants are part of the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative. Walter Koroshetz, director of NIH's National Institute of Neurological Disorders and Stroke said, "Neuroscience is rapidly moving toward a new frontier of research on human brains that may have long-lasting and unforeseen effects. These new awards signal our commitment to research conducted in a responsible way as to anticipate all potential consequences, and to ensure that research subjects have a clear understanding of the potential benefits and risks of participating in studies."
Dr. Lazaro-Munoz's Center was awarded one of the grants to identify and evaluate the ethical, legal and social concerns with adaptive deep brain stimulation (aDBS) technologies. Adaptive DBS is a relatively new version of the technology that enables recording of brain cell activity that is then used to regulate the brain in real time. He and his team will closely observe researchers conducting aDBS studies and administering in-depth interviews to trial participants, their caregivers, and researchers, as well as individuals who declined to participate in such studies. The goal is to gain a better understanding of the ethical concerns at stake in order to guide responsible research.
Dr. Lazaro-Munoz said one of the concerns is dehumanization. "By using this technology are we compromising what makes us human? When we are not in control of ourselves, are we ourselves?" He notes that similar concerns were raised about pharmaceutical treatments for illnesses. "Both change behaviors and emotional processing. However, there is a difference. Culturally we are more used to using drugs, not implanting devices into brain and computer interfaces. Many people think of it as science fiction."
The changes in behavior due to DBS can be dramatic, perhaps none more so than with Parkinson's disease; patients may see their chronic tremors suddenly vanish.
Pills for OCD and depression take longer than DBS to see significant improvement, sometimes months. "A DBS device is either on or off. And patients and families see changes immediately," Dr. Lazaro-Munoz said. "Family members are often startled by these changes, as are the patients." He's observed that patients feel more in control with pills because they can alter and "play" with the dose or even skip a dose.
The changes in behavior due to DBS can be dramatic, perhaps none more so than with Parkinson's disease; patients may see their chronic tremors suddenly vanish, like in this must-see video.
But surgical procedures to treat motor symptoms are also increasingly being implicated as a cause of behavioral changes, both positive and negative, in patients with Parkinson's. The personality changes reported in patients who undergo DBS include hypermania, pathological gambling, hypersexuality, impulsivity and aggressiveness. One patient who suffered from OCD fell in love with the music of Johnny Cash when his brain was stimulated. On the positive side, patients report memory enhancement.
One patient who is pleased with DBS is Greg Barstead, who was diagnosed with Parkinson's in 2003, when he was the president of Colonial Penn Life Insurance Company. He also has dystonia, which affects his neck and shoulders. Barstead said that DBS has been helpful for a range of symptoms: "My shoulder is a lot less stiff and my neck hurts less. And my tremors are under control. It is not perfect, as it doesn't relieve all the Parkinson's symptoms, but it does enough of a good job that both my wife and I are very happy I had DBS."
"We are not exactly sure what part of the brain causes depression. Doctors have not identified where to implant the device."
He said he hasn't noticed any personality changes, but noted that the disease itself can cause such changes. In fact, studies have shown that it can cause many psychiatric problems including depression and hallucinations. And, approximately a third of Parkinson's patients develop dementia.
Arthur L. Caplan, founding head of the Division of Medical Ethics at NYU School of Medicine, notes that unlike psychosurgery, DBS can be turned on and off and the device can be removed. "There are less ethical concerns around treating patients with Parkinson's disease than other illnesses because surgeons know exactly where to implant the device and have many years of experience with it," he said, adding that he is concerned about using DBS for other illnesses, such as depression. "We are not exactly sure what part of the brain causes depression. Doctors have not identified where to implant the device. And I would certainly not advocate its use in patients with mild depression."
Dr. Lazaro-Munoz said of the personality changes possible with DBS, physicians need to consider how the patients were functioning without it. "Patients who are candidates for DBS typically used many medications as well as psychotherapy before opting for DBS," he explained. "To me, the question is what is the net result of using this technology? Does the patient have regrets? Are the changes in personality significant or not? Although most DBS patients report being happy they underwent the procedure, some say they don't feel like themselves after DBS. Others feel they are more like themselves, especially if there are dramatic improvements in movement problems or relief of OCD symptoms."
And then there is the question of money. The costs of DBS are covered by most insurance companies and Medicare only for FDA-approved targets like Parkinson's. Off-label uses are not covered, at least for now.
Caplan reminds people that DBS devices are manufactured by companies that are interested in making money and the average cost per treatment is around $50,000. "I am interested in seeing DBS move forward," he said. "But we must be careful and not allow industry to make it go too fast, or be used on too many people, before we know it is effective."
Urinary tract infections account for more than 8 million trips to the doctor each year.
Few things are more painful than a urinary tract infection (UTI). Common in men and women, these infections account for more than 8 million trips to the doctor each year and can cause an array of uncomfortable symptoms, from a burning feeling during urination to fever, vomiting, and chills. For an unlucky few, UTIs can be chronic—meaning that, despite treatment, they just keep coming back.
But new research, presented at the European Association of Urology (EAU) Congress in Paris this week, brings some hope to people who suffer from UTIs.
Clinicians from the Royal Berkshire Hospital presented the results of a long-term, nine-year clinical trial where 89 men and women who suffered from recurrent UTIs were given an oral vaccine called MV140, designed to prevent the infections. Every day for three months, the participants were given two sprays of the vaccine (flavored to taste like pineapple) and then followed over the course of nine years. Clinicians analyzed medical records and asked the study participants about symptoms to check whether any experienced UTIs or had any adverse reactions from taking the vaccine.
The results showed that across nine years, 48 of the participants (about 54%) remained completely infection-free. On average, the study participants remained infection free for 54.7 months—four and a half years.
“While we need to be pragmatic, this vaccine is a potential breakthrough in preventing UTIs and could offer a safe and effective alternative to conventional treatments,” said Gernot Bonita, Professor of Urology at the Alta Bro Medical Centre for Urology in Switzerland, who is also the EAU Chairman of Guidelines on Urological Infections.
The news comes as a relief not only for people who suffer chronic UTIs, but also to doctors who have seen an uptick in antibiotic-resistant UTIs in the past several years. Because UTIs usually require antibiotics, patients run the risk of developing a resistance to the antibiotics, making infections more difficult to treat. A preventative vaccine could mean less infections, less antibiotics, and less drug resistance overall.
“Many of our participants told us that having the vaccine restored their quality of life,” said Dr. Bob Yang, Consultant Urologist at the Royal Berkshire NHS Foundation Trust, who helped lead the research. “While we’re yet to look at the effect of this vaccine in different patient groups, this follow-up data suggests it could be a game-changer for UTI prevention if it’s offered widely, reducing the need for antibiotic treatments.”
MILESTONE: Doctors have transplanted a pig organ into a human for the first time in history
A surgeon at Massachusetts General Hospital prepares a pig organ for transplant.
Surgeons at Massachusetts General Hospital made history last week when they successfully transplanted a pig kidney into a human patient for the first time ever.
The recipient was a 62-year-old man named Richard Slayman who had been living with end-stage kidney disease caused by diabetes. While Slayman had received a kidney transplant in 2018 from a human donor, his diabetes ultimately caused the kidney to fail less than five years after the transplant. Slayman had undergone dialysis ever since—a procedure that uses an artificial kidney to remove waste products from a person’s blood when the kidneys are unable to—but the dialysis frequently caused blood clots and other complications that landed him in the hospital multiple times.
As a last resort, Slayman’s kidney specialist suggested a transplant using a pig kidney provided by eGenesis, a pharmaceutical company based in Cambridge, Mass. The highly experimental surgery was made possible with the Food and Drug Administration’s “compassionate use” initiative, which allows patients with life-threatening medical conditions access to experimental treatments.
The new frontier of organ donation
Like Slayman, more than 100,000 people are currently on the national organ transplant waiting list, and roughly 17 people die every day waiting for an available organ. To make up for the shortage of human organs, scientists have been experimenting for the past several decades with using organs from animals such as pigs—a new field of medicine known as xenotransplantation. But putting an animal organ into a human body is much more complicated than it might appear, experts say.
“The human immune system reacts incredibly violently to a pig organ, much more so than a human organ,” said Dr. Joren Madsen, director of the Mass General Transplant Center. Even with immunosuppressant drugs that suppress the body’s ability to reject the transplant organ, Madsen said, a human body would reject an animal organ “within minutes.”
So scientists have had to use gene-editing technology to change the animal organs so that they would work inside a human body. The pig kidney in Slayman’s surgery, for instance, had been genetically altered using CRISPR-Cas9 technology to remove harmful pig genes and add human ones. The kidney was also edited to remove pig viruses that could potentially infect a human after transplant.
With CRISPR technology, scientists have been able to prove that interspecies organ transplants are not only possible, but may be able to successfully work long term, too. In the past several years, scientists were able to transplant a pig kidney into a monkey and have the monkey survive for more than two years. More recently, doctors have transplanted pig hearts into human beings—though each recipient of a pig heart only managed to live a couple of months after the transplant. In one of the patients, researchers noted evidence of a pig virus in the man’s heart that had not been identified before the surgery and could be a possible explanation for his heart failure.
So far, so good
Slayman and his medical team ultimately decided to pursue the surgery—and the risk paid off. When the pig organ started producing urine at the end of the four-hour surgery, the entire operating room erupted in applause.
Slayman is currently receiving an infusion of immunosuppressant drugs to prevent the kidney from being rejected, while his doctors monitor the kidney’s function with frequent ultrasounds. Slayman is reported to be “recovering well” at Massachusetts General Hospital and is expected to be discharged within the next several days.