Eight Big Medical and Science Trends to Watch in 2021

Promising developments underway include advancements in gene and cell therapy, better testing for COVID, and a renewed focus on climate change.
The world as we know it has forever changed. With a greater focus on science and technology than before, experts in the biotech and life sciences spaces are grappling with what comes next as SARS-CoV-2, the coronavirus that causes the COVID-19 illness, has spread and mutated across the world.
Even with vaccines being distributed, so much still remains unknown.
Jared Auclair, Technical Supervisor for the Northeastern University's Life Science Testing Center in Burlington, Massachusetts, guides a COVID testing lab that cranks out thousands of coronavirus test results per day. His lab is also focused on monitoring the quality of new cell and gene therapy products coming to the market.
Here are trends Auclair and other experts are watching in 2021.
Better Diagnostic Testing for COVID
Expect improvements in COVID diagnostic testing and the ability to test at home.
There are currently three types of coronavirus tests. The molecular test—also known as the RT-PCR test, detects the virus's genetic material, and is highly accurate, but it can take days to receive results. There are also antibody tests, done through a blood draw, designed to test whether you've had COVID in the past. Finally, there's the quick antigen test that isn't as accurate as the PCR test, but can identify if people are going to infect others.
Last month, Lucira Health secured the U.S. FDA Emergency Use Authorization for the first prescription molecular diagnostic test for COVID-19 that can be performed at home. On December 15th, the Ellume Covid-19 Home Test received authorization as the first over-the-counter COVID-19 diagnostic antigen test that can be done at home without a prescription. The test uses a nasal swab that is connected to a smartphone app and returns results in 15-20 minutes. Similarly, the BinaxNOW COVID-19 Ag Card Home Test received authorization on Dec. 16 for its 15-minute antigen test that can be used within the first seven days of onset of COIVD-19 symptoms.
Home testing has the possibility to impact the pandemic pretty drastically, Auclair says, but there are other considerations: the type and timing of test that is administered, how expensive is the test (and if it is financially feasible for the general public) and the ability of a home test taker to accurately administer the test.
"The vaccine roll-out will not eliminate the need for testing until late 2021 or early 2022."
Ideally, everyone would frequently get tested, but that would mean the cost of a single home test—which is expected to be around $30 or more—would need to be much cheaper, more in the $5 range.
Auclair expects "innovations in the diagnostic space to explode" with the need for more accurate, inexpensive, quicker COVID tests. Auclair foresees innovations to be at first focused on COVID point-of-care testing, but he expects improvements within diagnostic testing for other types of viruses and diseases too.
"We still need more testing to get the pandemic under control, likely over the next 12 months," Auclair says. "The vaccine roll-out will not eliminate the need for testing until late 2021 or early 2022."
Rise of mRNA-based Vaccines and Therapies
A year ago, vaccines weren't being talked about like they are today.
"But clearly vaccines are the talk of the town," Auclair says. "The reason we got a vaccine so fast was there was so much money thrown at it."
A vaccine can take more than 10 years to fully develop, according to the World Economic Forum. Prior to the new COVID vaccines, which were remarkably developed and tested in under a year, the fastest vaccine ever made was for mumps -- and it took four years.
"Normally you have to produce a protein. This is typically done in eggs. It takes forever," says Catherine Dulac, a neuroscientist and developmental biologist at Harvard University who won the 2021 Breakthrough Prize in Life Sciences. "But an mRNA vaccine just enabled [us] to skip all sorts of steps [compared with burdensome conventional manufacturing] and go directly to a product that can be injected into people."
Non-traditional medicines based on genetic research are in their infancy. With mRNA-based vaccines hitting the market for the first time, look for more vaccines to be developed for whatever viruses we don't currently have vaccines for, like dengue virus and Ebola, Auclair says.
"There's a whole bunch of things that could be explored now that haven't been thought about in the past," Auclair says. "It could really be a game changer."
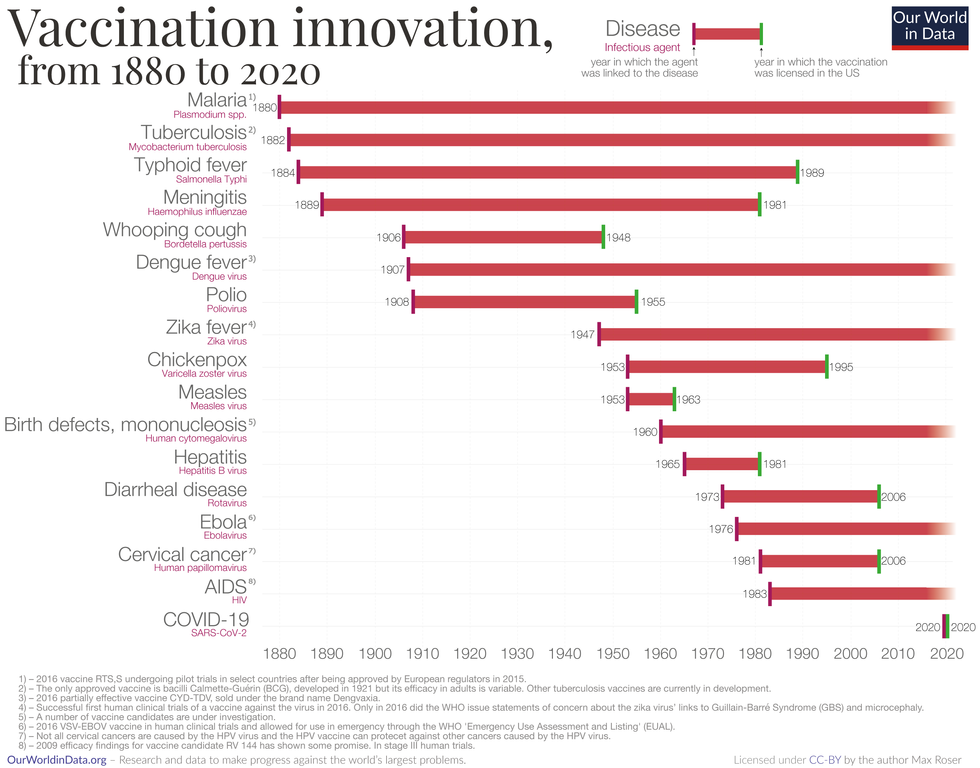
Vaccine Innovation over the last 140 years.
Max Roser/Our World in Data (Creative Commons license)
Advancements in Cell and Gene Therapies
CRISPR, a type of gene editing, is going to be huge in 2021, especially after the Nobel Prize in Chemistry was awarded to Emmanuelle Charpentier and Jennifer Doudna in October for pioneering the technology.
Right now, CRISPR isn't completely precise and can cause deletions or rearrangements of DNA.
"It's definitely not there yet, but over the next year it's going to get a lot closer and you're going to have a lot of momentum in this space," Auclair says. "CRISPR is one of the technologies I'm most excited about and 2021 is the year for it."
Gene therapies are typically used on rare genetic diseases. They work by replacing the faulty dysfunctional genes with corrected DNA codes.
"Cell and gene therapies are really where the field is going," Auclair says. "There is so much opportunity....For the first time in our life, in our existence as a species, we may actually be able to cure disease by using [techniques] like gene editing, where you cut in and out of pieces of DNA that caused a disease and put in healthy DNA," Auclair says.
For example, Spinal Muscular Atrophy is a rare genetic disorder that leads to muscle weakness, paralysis and death in children by age two. As of last year, afflicted children can take a gene therapy drug called Zolgensma that targets the missing or nonworking SMN1 gene with a new copy.
Another recent breakthrough uses gene editing for sickle cell disease. Victoria Gray, a mom from Mississippi who was exclusively followed by NPR, was the first person in the United States to be successfully treated for the genetic disorder with the help of CRISPR. She has continued to improve since her landmark treatment on July 2, 2019 and her once-debilitating pain has greatly eased.
"This is really a life-changer for me," she told NPR. "It's magnificent."
"You are going to see bigger leaps in gene therapies."
Look out also for improvements in cell therapies, but on a much lesser scale.
Cell therapies remove immune cells from a person or use cells from a donor. The cells are modified or cultured in lab, multiplied by the millions and then injected back into patients. These include stem cell therapies as well as CAR-T cell therapies, which are typically therapies of last resort and used in cancers like leukemia, Auclair says.
"You are going to see bigger leaps in gene therapies," Auclair says. "It's being heavily researched and we understand more about how to do gene therapies. Cell therapies will lie behind it a bit because they are so much more difficult to work with right now."
More Monoclonal Antibody Therapies
Look for more customized drugs to personalize medicine even more in the biotechnology space.
In 2019, the FDA anticipated receiving more than 200 Investigational New Drug (IND) applications in 2020. But with COVID, the number of INDs skyrocketed to 6,954 applications for the 2020 fiscal year, which ended September 30, 2020, according to the FDA's online tracker. Look for antibody therapies to play a bigger role.
Monoclonal antibodies are lab-grown proteins that mimic or enhance the immune system's response to fight off pathogens, like viruses, and they've been used to treat cancer. Now they are being used to treat patients with COVID-19.
President Donald Trump received a monoclonal antibody cocktail, called REGEN-COV2, which later received FDA emergency use authorization.
A newer type of monoclonal antibody therapy is Antibody-Drug Conjugates, also called ADCs. It's something we're going to be hearing a lot about in 2021, Auclair says.
"Antibody-Drug Conjugates is a monoclonal antibody with a chemical, we consider it a chemical warhead on it," Auclair says. "The monoclonal antibody binds to a specific antigen in your body or protein and delivers a chemical to that location and kills the infected cell."
Moving Beyond Male-Centric Lab Testing
Scientific testing for biology has, until recently, focused on testing males. Dulac, a Howard Hughes Medical Investigator and professor of molecular and cellular biology at Harvard University, challenged that idea to find brain circuitry behind sex-specific behaviors.
"For the longest time, until now, all the model systems in biology, are male," Dulac says. "The idea is if you do testing on males, you don't need to do testing on females."
Clinical models are done in male animals, as well as fundamental research. Because biological research is always done on male models, Dulac says the outcomes and understanding in biology is geared towards understanding male biology.
"All the drugs currently on the market and diagnoses of diseases are biased towards the understanding of male biology," Dulac says. "The diagnostics of diseases is way weaker in women than men."
That means the treatment isn't necessarily as good for women as men, she says, including what is known and understood about pain medication.
"So pain medication doesn't work well in women," Dulac says. "It works way better in men. It's true for almost all diseases that I know. Why? because you have a science that is dominated by males."
Although some in the scientific community challenge that females are not interesting or too complicated with their hormonal variations, Dulac says that's simply not true.
"There's absolutely no reason to decide 50% of life forms are interesting and the other 50% are not interesting. What about looking at both?" says Dulac, who was awarded the $3 million Breakthrough Prize in Life Sciences in September for connecting specific neural mechanisms to male and female parenting behaviors.
Disease Research on Single Cells
To better understand how diseases manifest in the body's cell and tissues, many researchers are looking at single-cell biology. Cells are the most fundamental building blocks of life. Much still needs to be learned.
"A remarkable development this year is the massive use of analysis of gene expression and chromosomal regulation at the single-cell level," Dulac says.
Much is focused on the Human Cell Atlas (HCA), a global initiative to map all cells in healthy humans and to better identify which genes associated with diseases are active in a person's body. Most estimates put the number of cells around 30 trillion.
Dulac points to work being conducted by the Cell Census Network (BICCN) Brain Initiative, an initiative by the National Institutes of Health to come up with an atlas of cell types in mouse, human and non-human primate brains, and the Chan Zuckerberg Initiative's funding of single-cell biology projects, including those focused on single-cell analysis of inflammation.
"Our body and our brain are made of a large number of cell types," Dulac says. "The ability to explore and identify differences in gene expression and regulation in massively multiplex ways by analyzing millions of cells is extraordinarily important."
Converting Plastics into Food
Yep, you heard it right, plastics may eventually be turned into food. The Defense Advanced Research Projects Agency, better known as DARPA, is funding a project—formally titled "Production of Macronutrients from Thermally Oxo-Degraded Wastes"—and asking researchers how to do this.
"When I first heard about this challenge, I thought it was absolutely absurd," says Dr. Robert Brown, director of the Bioeconomy Institute at Iowa State University and the project's principal investigator, who is working with other research partners at the University of Delaware, Sandia National Laboratories, and the American Institute of Chemical Engineering (AIChE)/RAPID Institute.
But then Brown realized plastics will slowly start oxidizing—taking in oxygen—and microorganisms can then consume it. The oxidation process at room temperature is extremely slow, however, which makes plastics essentially not biodegradable, Brown says.
That changes when heat is applied at brick pizza oven-like temperatures around 900-degrees Fahrenheit. The high temperatures get compounds to oxidize rapidly. Plastics are synthetic polymers made from petroleum—large molecules formed by linking many molecules together in a chain. Heated, these polymers will melt and crack into smaller molecules, causing them to vaporize in a process called devolatilization. Air is then used to cause oxidation in plastics and produce oxygenated compounds—fatty acids and alcohols—that microorganisms will eat and grow into single-cell proteins that can be used as an ingredient or substitute in protein-rich foods.
"The caveat is the microorganisms must be food-safe, something that we can consume," Brown says. "Like supplemental or nutritional yeast, like we use to brew beer and to make bread or is used in Australia to make Vegemite."
What do the microorganisms look like? For any home beer brewers, it's the "gunky looking stuff you'd find at the bottom after the fermentation process," Brown says. "That's cellular biomass. Like corn grown in the field, yeast or other microorganisms like bacteria can be harvested as macro-nutrients."
Brown says DARPA's ReSource program has challenged all the project researchers to find ways for microorganisms to consume any plastics found in the waste stream coming out of a military expeditionary force, including all the packaging of food and supplies. Then the researchers aim to remake the plastic waste into products soldiers can use, including food. The project is in the first of three phases.
"We are talking about polyethylene, polypropylene, like PET plastics used in water bottles and converting that into macronutrients that are food," says Brown.
Renewed Focus on Climate Change
The Union of Concerned Scientists say carbon dioxide levels are higher today than any point in at least 800,000 years.
"Climate science is so important for all of humankind. It is critical because the quality of life of humans on the planet depends on it."
Look for technology to help locate large-scale emitters of carbon dioxide, including sensors on satellites and artificial intelligence to optimize energy usage, especially in data centers.
Other technologies focus on alleviating the root cause of climate change: emissions of heat-trapping gasses that mainly come from burning fossil fuels.
Direct air carbon capture, an emerging effort to capture carbon dioxide directly from ambient air, could play a role.
The technology is in the early stages of development and still highly uncertain, says Peter Frumhoff, director of science and policy at Union of Concerned Scientists. "There are a lot of questions about how to do that at sufficiently low costs...and how to scale it up so you can get carbon dioxide stored in the right way," he says, and it can be very energy intensive.
One of the oldest solutions is planting new forests, or restoring old ones, which can help convert carbon dioxide into oxygen through photosynthesis. Hence the Trillion Trees Initiative launched by the World Economic Forum. Trees are only part of the solution, because planting trees isn't enough on its own, Frumhoff says. That's especially true, since 2020 was the year that human-made, artificial stuff now outweighs all life on earth.
More research is also going into artificial photosynthesis for solar fuels. The U.S. Department of Energy awarded $100 million in 2020 to two entities that are conducting research. Look also for improvements in battery storage capacity to help electric vehicles, as well as back-up power sources for solar and wind power, Frumhoff says.
Another method to combat climate change is solar geoengineering, also called solar radiation management, which reflects sunlight back to space. The idea stems from a volcanic eruption in 1991 that released a tremendous amount of sulfate aerosol particles into the stratosphere, reflecting the sunlight away from Earth. The planet cooled by a half degree for nearly a year, Frumhoff says. However, he acknowledges, "there's a lot of things we don't know about the potential impacts and risks" involved in this controversial approach.
Whatever the approach, scientific solutions to climate change are attracting renewed attention. Under President Trump, the White House Office of Science and Technology Policy didn't have an acting director for almost two years. Expect that to change when President-elect Joe Biden takes office.
"Climate science is so important for all of humankind," Dulac says. "It is critical because the quality of life of humans on the planet depends on it."
A new type of cancer therapy is shrinking deadly brain tumors with just one treatment
MRI scans after a new kind of immunotherapy for brain cancer show remarkable progress in one patient just days after the first treatment.
Few cancers are deadlier than glioblastomas—aggressive and lethal tumors that originate in the brain or spinal cord. Five years after diagnosis, less than five percent of glioblastoma patients are still alive—and more often, glioblastoma patients live just 14 months on average after receiving a diagnosis.
But an ongoing clinical trial at Mass General Cancer Center is giving new hope to glioblastoma patients and their families. The trial, called INCIPIENT, is meant to evaluate the effects of a special type of immune cell, called CAR-T cells, on patients with recurrent glioblastoma.
How CAR-T cell therapy works
CAR-T cell therapy is a type of cancer treatment called immunotherapy, where doctors modify a patient’s own immune system specifically to find and destroy cancer cells. In CAR-T cell therapy, doctors extract the patient’s T-cells, which are immune system cells that help fight off disease—particularly cancer. These T-cells are harvested from the patient and then genetically modified in a lab to produce proteins on their surface called chimeric antigen receptors (thus becoming CAR-T cells), which makes them able to bind to a specific protein on the patient’s cancer cells. Once modified, these CAR-T cells are grown in the lab for several weeks so that they can multiply into an army of millions. When enough cells have been grown, these super-charged T-cells are infused back into the patient where they can then seek out cancer cells, bind to them, and destroy them. CAR-T cell therapies have been approved by the US Food and Drug Administration (FDA) to treat certain types of lymphomas and leukemias, as well as multiple myeloma, but haven’t been approved to treat glioblastomas—yet.
CAR-T cell therapies don’t always work against solid tumors, such as glioblastomas. Because solid tumors contain different kinds of cancer cells, some cells can evade the immune system’s detection even after CAR-T cell therapy, according to a press release from Massachusetts General Hospital. For the INCIPIENT trial, researchers modified the CAR-T cells even further in hopes of making them more effective against solid tumors. These second-generation CAR-T cells (called CARv3-TEAM-E T cells) contain special antibodies that attack EFGR, a protein expressed in the majority of glioblastoma tumors. Unlike other CAR-T cell therapies, these particular CAR-T cells were designed to be directly injected into the patient’s brain.
The INCIPIENT trial results
The INCIPIENT trial involved three patients who were enrolled in the study between March and July 2023. All three patients—a 72-year-old man, a 74-year-old man, and a 57-year-old woman—were treated with chemo and radiation and enrolled in the trial with CAR-T cells after their glioblastoma tumors came back.
The results, which were published earlier this year in the New England Journal of Medicine (NEJM), were called “rapid” and “dramatic” by doctors involved in the trial. After just a single infusion of the CAR-T cells, each patient experienced a significant reduction in their tumor sizes. Just two days after receiving the infusion, the glioblastoma tumor of the 72-year-old man decreased by nearly twenty percent. Just two months later the tumor had shrunk by an astonishing 60 percent, and the change was maintained for more than six months. The most dramatic result was in the 57-year-old female patient, whose tumor shrank nearly completely after just one infusion of the CAR-T cells.
The results of the INCIPIENT trial were unexpected and astonishing—but unfortunately, they were also temporary. For all three patients, the tumors eventually began to grow back regardless of the CAR-T cell infusions. According to the press release from MGH, the medical team is now considering treating each patient with multiple infusions or prefacing each treatment with chemotherapy to prolong the response.
While there is still “more to do,” says co-author of the study neuro-oncologist Dr. Elizabeth Gerstner, the results are still promising. If nothing else, these second-generation CAR-T cell infusions may someday be able to give patients more time than traditional treatments would allow.
“These results are exciting but they are also just the beginning,” says Dr. Marcela Maus, a doctor and professor of medicine at Mass General who was involved in the clinical trial. “They tell us that we are on the right track in pursuing a therapy that has the potential to change the outlook for this intractable disease.”
A recent study in The Lancet Oncology showed that AI found 20 percent more cancers on mammogram screens than radiologists alone.
Since the early 2000s, AI systems have eliminated more than 1.7 million jobs, and that number will only increase as AI improves. Some research estimates that by 2025, AI will eliminate more than 85 million jobs.
But for all the talk about job security, AI is also proving to be a powerful tool in healthcare—specifically, cancer detection. One recently published study has shown that, remarkably, artificial intelligence was able to detect 20 percent more cancers in imaging scans than radiologists alone.
Published in The Lancet Oncology, the study analyzed the scans of 80,000 Swedish women with a moderate hereditary risk of breast cancer who had undergone a mammogram between April 2021 and July 2022. Half of these scans were read by AI and then a radiologist to double-check the findings. The second group of scans was read by two researchers without the help of AI. (Currently, the standard of care across Europe is to have two radiologists analyze a scan before diagnosing a patient with breast cancer.)
The study showed that the AI group detected cancer in 6 out of every 1,000 scans, while the radiologists detected cancer in 5 per 1,000 scans. In other words, AI found 20 percent more cancers than the highly-trained radiologists.

But even though the AI was better able to pinpoint cancer on an image, it doesn’t mean radiologists will soon be out of a job. Dr. Laura Heacock, a breast radiologist at NYU, said in an interview with CNN that radiologists do much more than simply screening mammograms, and that even well-trained technology can make errors. “These tools work best when paired with highly-trained radiologists who make the final call on your mammogram. Think of it as a tool like a stethoscope for a cardiologist.”
AI is still an emerging technology, but more and more doctors are using them to detect different cancers. For example, researchers at MIT have developed a program called MIRAI, which looks at patterns in patient mammograms across a series of scans and uses an algorithm to model a patient's risk of developing breast cancer over time. The program was "trained" with more than 200,000 breast imaging scans from Massachusetts General Hospital and has been tested on over 100,000 women in different hospitals across the world. According to MIT, MIRAI "has been shown to be more accurate in predicting the risk for developing breast cancer in the short term (over a 3-year period) compared to traditional tools." It has also been able to detect breast cancer up to five years before a patient receives a diagnosis.
The challenges for cancer-detecting AI tools now is not just accuracy. AI tools are also being challenged to perform consistently well across different ages, races, and breast density profiles, particularly given the increased risks that different women face. For example, Black women are 42 percent more likely than white women to die from breast cancer, despite having nearly the same rates of breast cancer as white women. Recently, an FDA-approved AI device for screening breast cancer has come under fire for wrongly detecting cancer in Black patients significantly more often than white patients.
As AI technology improves, radiologists will be able to accurately scan a more diverse set of patients at a larger volume than ever before, potentially saving more lives than ever.

