The science of slowing down aging - even if you're not a tech billionaire

Chris Mirabile sprints on a track in Sarasota, Florida, during his daily morning workout. He claims to be a superager already, at age 38, with test results to back it up.
Earlier this year, Harvard scientists reported that they used an anti-aging therapy to reverse blindness in elderly mice. Several other studies in the past decade have suggested that the aging process can be modified, at least in lab organisms. Considering mice and humans share virtually the same genetic makeup, what does the rodent-based study mean for the humans?
In truth, we don’t know. Maybe nothing.
What we do know, however, is that a growing number of people are dedicating themselves to defying the aging process, to turning back the clock – the biological clock, that is. Take Bryan Johnson, a man who is less mouse than human guinea pig. A very wealthy guinea pig.
The 45-year-old venture capitalist spends over $2 million per year reversing his biological clock. To do this, he employs a team of 30 medical doctors and other scientists. His goal is to eventually reset his biological clock to age 18, and “have all of his major organs — including his brain, liver, kidneys, teeth, skin, hair, penis and rectum — functioning as they were in his late teens,” according to a story earlier this year in the New York Post.
But his daily routine paints a picture that is far from appealing: for example, rigorously adhering to a sleep schedule of 8 p.m. to 5 a.m. and consuming more than 100 pills and precisely 1,977 calories daily. Considering all of Johnson’s sacrifices, one discovers a paradox:
To live forever, he must die a little every day until he reaches his goal - if he ever reaches his goal.
Less extreme examples seem more helpful for people interested in happy, healthy aging. Enter Chris Mirabile, a New Yorker who says on his website, SlowMyAge.com, that he successfully reversed his biological age by 13.6 years, from the chronological age of 37.2 to a biological age of 23.6. To put this achievement in perspective, Johnson, to date, has reversed his biological clock by 2.5 years.
Mirabile's habits and overall quest to turn back the clock trace back to a harrowing experience at age 16 during a school trip to Manhattan, when he woke up on the floor with his shirt soaked in blood.
Mirabile, who is now 38, supports his claim with blood tests that purport to measure biological age by assessing changes to a person’s epigenome, or the chemical marks that affect how genes are expressed. Mirabile’s tests have been run and verified independently by the same scientific lab that analyzes Johnson’s. (In an email to Leaps.org, the lab, TruDiagnostic, confirmed Mirabile’s claims about his test results.)
There is considerable uncertainty among scientists about the extent to which these tests can accurately measure biological age in individuals. Even so, Mirabile’s results are intriguing. They could reflect his smart lifestyle for healthy aging.
His habits and overall quest to turn back the clock trace back to a harrowing experience at age 16 during a school trip to Manhattan, when Mirabile woke up on the floor with his shirt soaked in blood. He’d severed his tongue after a seizure. He later learned it was caused by a tumor the size of a golf ball. As a result, “I found myself contemplating my life, what I had yet to experience, and mortality – a theme that stuck with me during my year of recovery and beyond,” Mirabile told me.
For the next 15 years, he researched health and biology, integrating his learnings into his lifestyle. Then, in his early 30s, he came across an article in the journal Cell, "The Hallmarks of Aging," that outlined nine mechanisms of the body that define the aging process. Although the paper says there are no known interventions to delay some of these mechanisms, others, such as inflammation, struck Mirabile as actionable. Reading the paper was his “moment of epiphany” when it came to the areas where he could assert control to maximize his longevity.
He also wanted “to create a resource that my family, friends, and community could benefit from in the short term,” he said. He turned this knowledge base into a company called NOVOS dedicated to extending lifespan.
His longevity advice is more accessible than Johnson’s multi-million dollar approach, as Mirabile spends a fraction of that amount. Mirabile takes one epigenetic test per year and has a gym membership at $45 per month. Unlike Johnson, who takes 100 pills per day, Mirabile takes 10, costing another $45 monthly, including a B-complex, fish oil, Vitamins D3 and K2, and two different multivitamin supplements.
Mirabile’s methods may be easier to apply in other ways as well, since they include activities that many people enjoy anyway. He’s passionate about outdoor activities, travels frequently, and has loving relationships with friends and family, including his girlfriend and collie.
Here are a few of daily routines that could, he thinks, contribute to his impressively young bio age:
After waking at 7:45 am, he immediately drinks 16 ounces of water, with 1/4 teaspoon of sodium and potassium to replenish electrolytes. He takes his morning vitamins, brushes and flosses his teeth, puts on a facial moisturizing sunblock and goes for a brisk, two-mile walk in the sun. At 8:30 am on Mondays, Wednesdays, and Fridays he lift weights, focusing on strength and power, especially in large muscle groups.
Tuesdays, Thursdays and Saturdays are intense cardio days. He runs 5-7 miles or bicycles for 60 minutes first thing in the morning at a brisk pace, listening to podcasts. Sunday morning cardio is more leisurely.
After working out each day, he’s back home at 9:20 am, where he makes black coffee, showers, then applies serum and moisturizing sunblock to his face. He works for about three hours on his laptop, then has a protein shake and fruit.
Mirabile is a dedicated intermittent faster, with a six hour eating window in between 18 hours fasts. At 3 pm, he has lunch. The Mediterranean lineup often features salmon, sardines, olive oil, pink Himalayan salt plus potassium salt for balance, and lots of dried herbs and spices. He almost always finishes with 1/3 to 1/2 bar of dark chocolate.
If you are what you eat, Mirabile is made of mostly plants and lean meats. He follows a Mediterranean diet full of vegetables, fruits, fatty fish and other meats full of protein and unsaturated fats. “These may cost more than a meal at an American fast-food joint, but then again, not by much,” he said. Each day, he spends $25 on all his meals combined.
At 6 pm, he takes the dog out for a two-mile walk, taking calls for work or from family members along the way. At 7 pm, he dines with his girlfriend. Like lunch, this meal is heavy on widely available ingredients, including fish, fresh garlic, and fermented food like kimchi. Mirabile finishes this meal with sweets, like coconut milk yogurt with cinnamon and clove, some stevia, a mix of fresh berries and cacao nibs.
If Mirabile's epigenetic tests are accurate, his young biological age could be thanks to his healthy lifestyle, or it could come from a stroke of luck if he inherited genes that protect against aging.
At 8 pm, he wraps up work duties and watches shows with his girlfriend, applies serum and moisturizer yet again, and then meditates with the lights off. This wind-down, he said, improves his sleep quality. Wearing a sleep mask and earplugs, he’s asleep by about 10:30.
“I’ve achieved stellar health outcomes, even after having had the physiological stressors of a brain tumor, without spending a fortune,” Mirabile said. “In fact, even during times when I wasn’t making much money as a startup founder with few savings, I still managed to live a very healthy, pro-longevity lifestyle on a modest budget.”
Mirabile said living a cleaner, healthier existence is a reality that many readers can achieve. It’s certainly true that many people live in food deserts and have limited time for exercise or no access to gyms, but James R. Doty, a clinical professor of neurosurgery at Stanford, thinks many can take more action to stack the odds that they’ll “be happy and live longer.” Many of his recommendations echo aspects of Mirabile’s lifestyle.
Each night, Doty said, it’s vital to get anywhere between 6-8 hours of good quality sleep. Those who sleep less than 6 hours per night are at an increased risk of developing a whole host of medical problems, including high blood pressure, type 2 diabetes, and stroke.
In addition, it’s critical to follow Mirabile’s prescription of exercise for about one hour each day, and intensity levels matter. Doty noted that, in 2017, researchers at Brigham Young University found that people who ran at a fast pace for 30-40 minutes five days per week were, on average, biologically younger by nine years, compared to those who subscribed to more moderate exercise programs, as well as those who rarely exercised.
When it comes to nutrition, one should consider fasting for 16 hours per day, Doty said. This is known as the 16/8 method, where one’s daily calories are consumed within an eight hour window, fasting for the remaining 16 hours, just like Mirabile. Intermittent fasting is associated with cellular repair and less inflammation, though it’s not for everyone, Doty added. Consult with a medical professional before trying a fasting regimen.
Finally, Doty advised to “avoid anger, avoid stress.” Easier said than done, but not impossible. “Between stimulus and response, there is a pause and within that pause lies your freedom,” Doty said. Mirabile’s daily meditation ritual could be key to lower stress for healthy aging. Research has linked regular, long-term meditation to having a lower epigenetic age, compared to control groups.
Many other factors could apply. Having a life purpose, as Mirabile does with his company, has also been associated with healthy aging and lower epigenetic age. Of course, Mirabile is just one person, so it’s hard to know how his experience will apply to others. If his tests are accurate, his young biological age could be thanks to his healthy lifestyle, or it could come from a stroke of luck if he inherited genes that protect against aging. Clearly, though, any such genes did not protect him from cancer at an early age.
The third and perhaps most likely explanation: Mirabile’s very young biological age results from a combination of these factors. Some research shows that genetics account for only 25 percent of longevity. That means environmental factors could be driving the other 75 percent, such as where you live, frequency of exercise, quality of nutrition and social support.
The middle-aged – even Brian Johnson – probably can’t ever be 18 again. But more modest goals are reasonable for many. Control what you can for a longer, healthier life.
With this new technology, hospitals and pharmacies could make vaccines and medicines onsite
New research focuses on methods that could change medicine-making worldwide. The scientists propose bursting cells open, removing their DNA and using the cellular gears inside to make therapies.
Most modern biopharmaceutical medicines are produced by workhorse cells—typically bacterial but sometimes mammalian. The cells receive the synthesizing instructions on a snippet of a genetic code, which they incorporate into their DNA. The cellular machinery—ribosomes, RNAs, polymerases, and other compounds—read and use these instructions to build the medicinal molecules, which are harvested and administered to patients.
Although a staple of modern pharma, this process is complex and expensive. One must first insert the DNA instructions into the cells, which they may or may not uptake. One then must grow the cells, keeping them alive and well, so that they produce the required therapeutics, which then must be isolated and purified. To make this at scale requires massive bioreactors and big factories from where the drugs are distributed—and may take a while to arrive where they’re needed. “The pandemic showed us that this method is slow and cumbersome,” says Govind Rao, professor of biochemical engineering who directs the Center for Advanced Sensor Technology at the University of Maryland, Baltimore County (UMBC). “We need better methods that can work faster and can work locally where an outbreak is happening.”
Rao and his team of collaborators, which spans multiple research institutions, believe they have a better approach that may change medicine-making worldwide. They suggest forgoing the concept of using living cells as medicine-producers. Instead, they propose breaking the cells and using the remaining cellular gears for assembling the therapeutic compounds. Instead of inserting the DNA into living cells, the team burst them open, and removed their DNA altogether. Yet, the residual molecular machinery of ribosomes, polymerases and other cogwheels still functioned the way it would in a cell. “Now if you drop your DNA drug-making instructions into that soup, this machinery starts making what you need,” Rao explains. “And because you're no longer worrying about living cells, it becomes much simpler and more efficient.” The collaborators detail their cell-free protein synthesis or CFPS method in their recent paper published in preprint BioAxiv.
While CFPS does not use living cells, it still needs the basic building blocks to assemble proteins from—such as amino acids, nucleotides and certain types of enzymes. These are regularly added into this “soup” to keep the molecular factory chugging. “We just mix everything in as a batch and we let it integrate,” says James Robert Swartz, professor of chemical engineering and bioengineering at Stanford University and co-author of the paper. “And we make sure that we provide enough oxygen.” Rao likens the process to making milk from milk powder.
For a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology.
The idea of a cell-free protein synthesis is older than one might think. Swartz first experimented with it around 1997, when he was a chemical engineer at Genentech. While working on engineering bacteria to make pharmaceuticals, he discovered that there was a limit to what E. coli cells, the workhorse darling of pharma, could do. For example, it couldn’t grow and properly fold some complex proteins. “We tried many genetic engineering approaches, many fermentation, development, and environmental control approaches,” Swartz recalls—to no avail.
“The organism had its own agenda,” he quips. “And because everything was happening within the organism, we just couldn't really change those conditions very easily. Some of them we couldn’t change at all—we didn’t have control.”
It was out of frustration with the defiant bacteria that a new idea took hold. Could the cells be opened instead, so that the protein-forming reactions could be influenced more easily? “Obviously, we’d lose the ability for them to reproduce,” Swartz says. But that also meant that they no longer needed to keep the cells alive and could focus on making the specific reactions happen. “We could take the catalysts, the enzymes, and the more complex catalysts and activate them, make them work together, much as they would in a living cell, but the way we wanted.”
In 1998, Swartz joined Stanford, and began perfecting the biochemistry of the cell-free method, identifying the reactions he wanted to foster and stopping those he didn’t want. He managed to make the idea work, but for a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology. For their BioArxiv paper, the team tested the method by growing a specific antiviral protein called griffithsin.
First identified by Barry O’Keefe at National Cancer Institute over a decade ago, griffithsin is an antiviral known to interfere with many viruses’ ability to enter cells—including HIV, SARS, SARS-CoV-2, MERS and others. Originally isolated from the red algae Griffithsia, it works differently from antibodies and antibody cocktails.
Most antiviral medicines tend to target the specific receptors that viruses use to gain entry to the cells they infect. For example, SARS-CoV-2 uses the infamous spike protein to latch onto the ACE2 receptor of mammalian cells. The antibodies or other antiviral molecules stick to the spike protein, shutting off its ability to cling onto the ACE2 receptors. Unfortunately, the spike proteins mutate very often, so the medicines lose their potency. On the contrary, griffithsin has the ability to cling to the different parts of viral shells called capsids—namely to the molecules of mannose, a type of sugar. That extra stuff, glued all around the capsid like dead weight, makes it impossible for the virus to squeeze into the cell.
“Every time we have a vaccine or an antibody against a specific SARS-CoV-2 strain, that strain then mutates and so you lose efficacy,” Rao explains. “But griffithsin molecules glom onto the viral capsid, so the capsid essentially becomes a sticky mess and can’t enter the cell.” Mannose molecules also don’t mutate as easily as viruses’ receptors, so griffithsin-based antivirals do not have to be constantly updated. And because mannose molecules are found on many viruses’ capsids, it makes griffithsin “a universal neutralizer,” Rao explains.
“When griffithsin was discovered, we recognized that it held a lot of promise as a potential antiviral agent,” O’Keefe says. In 2010, he published a paper about griffithsin efficacy in neutralizing viruses of the corona family—after the first SARS outbreak in the early 2000s, the scientific community was interested in such antivirals. Yet, griffithsin is still not available as an off-the-shelf product. So during the Covid pandemic, the team experimented with synthesizing griffithsin using the cell-free production method. They were able to generate potent griffithsin in less than 24 hours without having to grow living cells.
The antiviral protein isn't the only type of medicine that can be made cell-free. The proteins needed for vaccine production could also be made the same way. “Such portable, on-demand drug manufacturing platforms can produce antiviral proteins within hours, making them ideal for combating future pandemics,” Rao says. “We would be able to stop the pandemic before it spreads.”
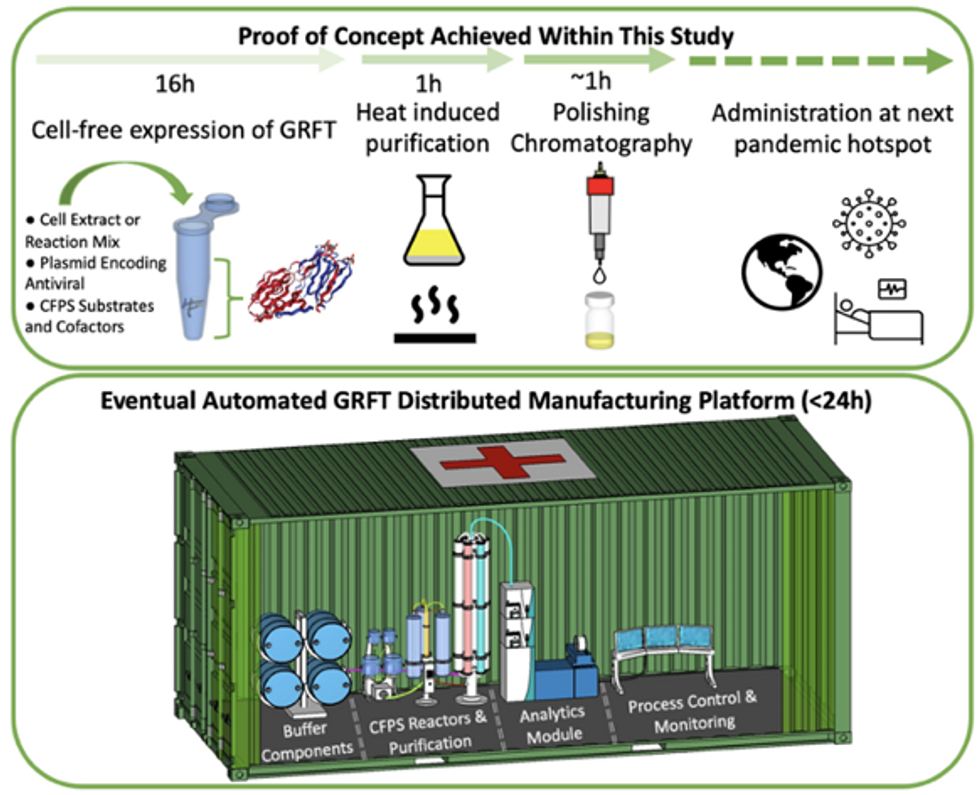
Top: Describes the process used in the study. Bottom: Describes how the new medicines and vaccines could be made at the site of a future viral outbreak.
Image courtesy of Rao and team, sourced from An approach to rapid distributed manufacturing of broad spectrumanti-viral griffithsin using cell-free systems to mitigate pandemics.
Rao’s idea is to perfect the technology to the point that any hospital or pharmacy can load up the media containing molecular factories, mix up the required amino acids, nucleotides and enzymes, and harvest the meds within hours. That will allow making medicines onsite and on demand. “That would be a self-contained production unit, so that you could just ship the production wherever the pandemic is breaking out,” says Swartz.
These units and the meds they produce, will, of course, have to undergo rigorous testing. “The biggest hurdles will be validating these against conventional technology,” Rao says. The biotech industry is risk-averse and prefers the familiar methods. But if this approach works, it may go beyond emergency situations and revolutionize the medicine-making paradigm even outside hospitals and pharmacies. Rao hopes that someday the method might become so mainstream that people may be able to buy and operate such reactors at home. “You can imagine a diabetic patient making insulin that way, or some other drugs,” Rao says. It would work not unlike making baby formula from the mere white powder. Just add water—and some oxygen, too.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
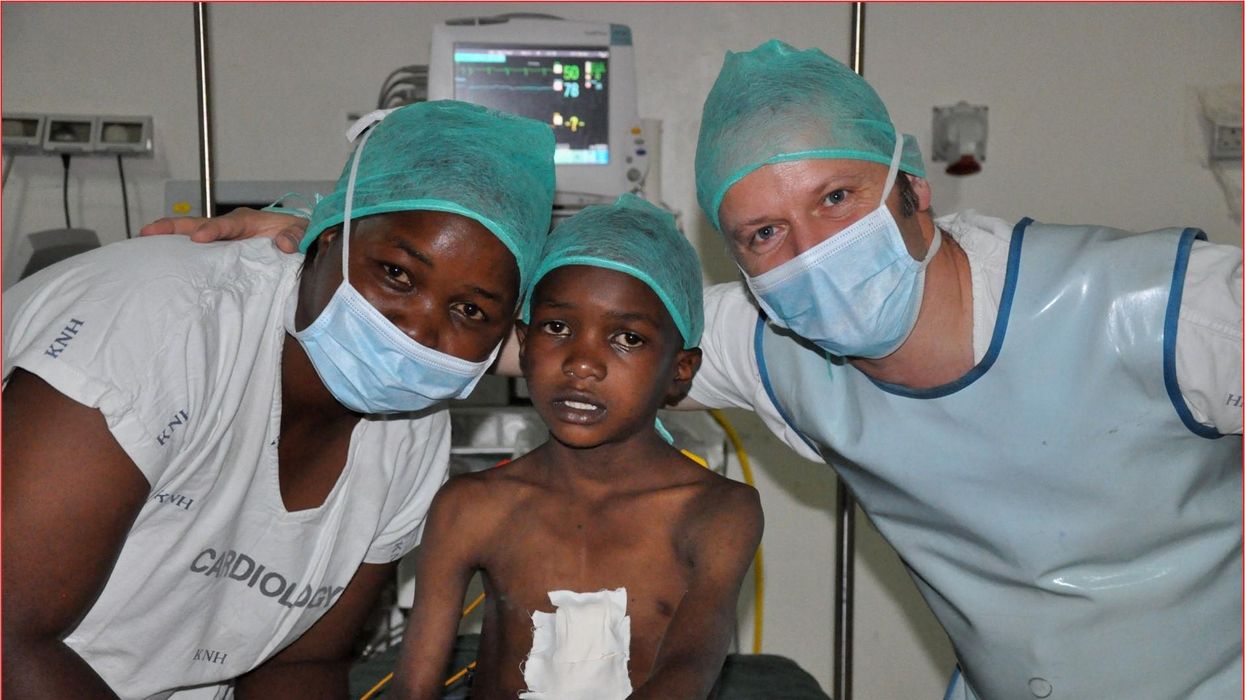
These doctors have a heart for recycling
In the U.S. and Europe, it is illegal to reuse pacemakers and other implants. Therefore, cardiologists export them to the global South where they save the lives of people of all ages.
This is part 3 of a three part series on a new generation of doctors leading the charge to make the health care industry more sustainable - for the benefit of their patients and the planet. Read part 1 here and part 2 here.
One could say that over 400 people owe their life to the fact that Carsten Israel fell in love. Twenty years ago, as a young doctor in Frankfurt, Germany, he began to court an au pair from Kenya, Elisabeth, his now-wife of 13 years with whom he has three children. When the couple started visiting her parents in Kenya, Israel wanted to check out the local hospitals, “just out of professional curiosity,“ says the cardiologist, who is currently the head doctor at the Clinic for Interior Medicine in Bielefeld. “I was completely shocked.“
Often he observed there were no doctors in the E.R.s, and hte nurses could render only basic first aid. “When somebody fell into a coma, they fell into a coma,“ Israel remembers. “There weren’t even any defibrillators to restart a patient’s heart,” while defibrillators are standard equipment in most clinics in the U.S. and Europe as lifesaving devices. When Israel finally visited the largest and most modern hospital in Nairobi, he found it better equipped but he learned that its services were only available to patients who could afford them. The cardiologist there had a drawer full of petitions from patients with heart ailments who couldn’t afford lifesaving surgery. Even two decades ago, a pacemaker cost $5,000 in Kenya, which made it unaffordable for most Kenyans who earn an average of $600 per month.
Since 2003, Israel and a team of two doctors and two nurses visit Kenya and Zambia once or twice a year to implant German pacemakers for free. Notably, the pacemakers and defibrillators Israel exports to Africa would end up in the landfill in Germany. Clinics have to pay for specialized services to dispose of this medical equipment. “In Germany, I could go to jail if I used a defibrillator that is one day past its expiration date,“ Israel says, “but in Kenya, people don’t have the money for the cheapest model. What nonsense to throw this precious medical equipment away while people in poorer countries die because they desperately need it.“
Israel works at the breakpoint between the laws in a wealthy country like Germany and the reality in the global South. The U.S. and most European countries have strict laws that ban the reuse of medical implants and enforce strict expiration dates for medical equipment. “But if a pacemaker is a few days past its expiration date, it still works perfectly fine,“ Israel says. “And it also happens that we implant a pacemaker and five months later it turns out that the patient needs a different kind. Then we replace it and we’d have to trash the first one in Germany, though it could easily run another 12 years.“
“If we get this right, we have lots of devices we can implant, hips and knees, etcetera. Where this will lead is limitless," says Eva Kline Rogers, the program coordinator for My Heart, Your Heart.
Israel has been collecting donations of pacemakers and defibrillators from manufacturers but also from other doctors and from funeral homes for his nonprofit Pacemakers for East Africa since 2003. Most funeral homes in the U.S. and Europe are legally obliged to remove pacemakers from the dead before cremation. “Most pacemakers survive their owners,“ says Israel. He sterilizes the pacemakers and finds them a new life in East Africa. Studies show that reused pacemakers carry no greater risk for the patients than new ones.
In the U.S., University of Michigan professor Thomas Crawford heads up a similar initiative, My Heart, Your Heart. “Each year 1 to 2 million individuals worldwide die due to a lack of access to pacemakers and defibrillators,” the organization notes on its website. The nonprofit was founded in 2009, but it took four years for the doctors to get permission from the FDA to export pacemakers. Since receiving permission, the organization has sent dozens of devices to the Philippines, Haiti, Venezuela, Kenya, Sierra Leone and Ukraine. “We were the first doctors ever to implant a pacemaker in Sierra Leone in 2018,” says Crawford, who has traveled extensively to most of the recipient countries.
Even individuals can donate their pacemakers; the organization offers a prepaid envelope. “My mother recently passed and she donated her device,” says Tina Alexandris-Souphis, one of the doctors at University of Michigan who collaborates on My Heart, Your Heart. The organization works with World Medical Relief and the U.K. based charity Pace4Life to maintain a registry of the most urgent patients and send devices to where they are needed the most.
My Heart, Your Heart is also conducting a randomized controlled trial to provide further evidence that reused pacemakers pose no additional risk. “Our vision is that we establish this is safe and create a blueprint for organizations around the world to safely reuse these devices instead of them being thrown in the trash,” says Eva Kline Rogers, the program’s coordinator. “If we get this right, we have lots of devices we can implant, hips and knees, etc. Where this will lead is limitless.” She points out that in addition to receiving the donated devices, the doctors in the global South also benefit from the expertise of renowned cardiologists, such as Crawford, who sometimes advise them in complex cases.
And Adrian Baranchuk, a Canadian doctor at the Kingston General Hospital at the Queen’s University, regularly travels through South America with his “cardiology van” to help villagers in remote areas with heart problems.
Israel says that he’s been accused of racism, in thinking that these pacemakers are suitable for those in the global South - many of whom are people of color - even though officials in wealthier countries consider them to be trash. The cardiologist counters such criticism with stories about desperate need of his patients. At his first medical visit to Nairobi that he organized with a local cardiologist, six patients were waiting for him. “In Germany, they would all be considered emergencies,” Israel says. One eighty-year old grandmother had a heartrate of 18. “I’ve never before seen anything like this,” Israel exclaims. “At first I thought I couldn’t find her pulse before I realized that her heart was only beating once every three seconds.” After the surgery, she got up, dressed herself and hurriedly packed her bag, explaining she had a ton of work to accomplish. Her family was in disbelief, Israel says. “They told me she had been bedridden for five years because as soon as she tried to get up she would faint.”

Israel has been accused of racism, in thinking that these pacemakers are suitable for those in the global South even though they're considered to be trash by officials in wealthier countries. The cardiologist counters such criticism with stories about desperate need of his patients.
Carsten Israel
The hospital in Nairobi where Israel conducts the surgeries, charges patients $200 for the use of its facilities. If patients can’t afford that sum, Israel will pay it from the funds of his nonprofit. For some people, $200 far exceeds their resources. Once, a family from the extremely poor Northern region of Kenya told him they couldn’t afford the $3 for the bus ride to Nairobi. Israel suspected this was a pretense because they were afraid of the surgery and agreed to reimburse the $3, “but when they came, they were wearing rags and were so rail-thin, I understood that they really needed every cent they had for food.”
Israel is a renowned cardiologists in Germany. And yet, he considers his work in East Africa to be particularly meaningful. “Generally, most patients in Germany will get the treatment they need,” he says, “and I never before experienced that people have an illness that is easily curable but simply won’t be treated.” He also feels a heavy responsibility. Many patients have his personal cell phone and call him when they have problems or good news about how they’re doing.
Some of those progress reports come much faster than in Israel’s home country. Before he implanted a pacemaker in a tall Massai in Kenya, the man had been picked on by his family because he wouldn’t help much with the hard work on the family peanut farm. “When I examined him, he had a pulse of 40,” Israel says. “It’s a miracle he was even standing upright, let alone hauling heavy bags.” After the surgery, Israel advised his patient to stay the night for observation, but the patient couldn’t wait to leave. Two hours later, he returned, covered in sweat. He’d been running sprints with his brothers to test the new device. Israel shakes his head. In Germany, it would be unthinkable for a patient to engage in athletics immediately after surgery. But the patient was exuberant: “I was the fastest!”
The success stories are notable partly because the challenges remain so steep. In Zambia, for instance, there is a single cardiologist; she determined to become one after losing her younger sister to an easily curable heart disease. Often, the hospitals not only lack pacemakers but also sterile surgery equipment, antibiotics and other essential material. Therefore, Israel and his team import everything they need for the surgeries, including medication. If necessary, they improvise. “I’ve done surgery with a desk lamp hanging from the ceiling by threads,” Israel says. He already knows that he will need to return to Kenya in six months to replace the pacemaker of one of his patients and replace the batteries in others. If he doesn’t travel, lives are at risk.