Fixing a Baby’s Abnormal Genes in the Womb May Soon Be Possible

A couple holds up a picture of their ultrasound.
By now you have probably heard something about CRISPR, the simple and relatively inexpensive method of precisely editing the genomes of plants, animals, and humans.
The treatment of disease in fetuses, the liminal category of life between embryos and humans, poses the next frontier.
Through CRISPR and other methods of gene editing, scientists have produced crops to be more nutritious, better able to resist pests, and tolerate droughts; engineered animals ranging from fruit flies to monkeys to make them better suited for scientific study; and experimentally treated the HIV virus, Hepatitis B, and leukemia in human patients.
There are also currently FDA-approved trials to treat blindness, cancer, and sickle cell disease in humans using gene editing, and there is consensus that CRISPR's therapeutic applications will grow significantly in the coming years.
While the treatment of human disease through use of gene editing is not without its medical and ethical concerns, the avoidance of disease in embryos is far more fraught. Nonetheless, Nature reported in November that He Jiankui, a scientist in China, had edited twin embryos to disable a gene called CCR5 in hopes of avoiding transmission of HIV from their HIV-positive father.
Though there are questions about the effectiveness and necessity of this therapy, He reported that sequencing has proven his embryonic gene edits were successful and the twins were "born normal and healthy," although his claims have not been independently verified.
More recently, Denis Rebrikov, a Russian scientist, announced his plans to disable the same gene in embryos to be implanted in HIV-positive women later this year. Futuristic as it may seem, prenatal gene editing is already here.
The treatment of disease in fetuses, the liminal category of life between embryos and humans, poses the next frontier. Numerous conditions—some minor, some resulting in a lifetime of medical treatment, some incompatible with life outside of the womb—can be diagnosed through use of prenatal diagnostic testing. There is promising research suggesting doctors will soon be able to treat or mitigate at least some of them through use of fetal gene editing.
This research could soon present women carrying genetically anomalous fetuses a third option aside from termination or birthing a child who will likely face a challenging and uncertain medical future: Whether to undergo a fetal genetic intervention.
However, genetic intervention will open the door to a host of ethical considerations, particularly with respect to the relationship between pregnant women and prenatal genetic counselors. Current counselors theoretically provide objective information and answer questions rather than advise their pregnant client whether to continue with her pregnancy, despite the risks, or to have an abortion.
In practice, though, prenatal genetic counseling is most often directive, and the nature of the counseling pregnant women receive can depend on numerous factors, including their religious and cultural beliefs, their perceived ability to handle a complicated pregnancy and subsequent birth, and their financial status. Introducing the possibility of a fetal genetic intervention will exacerbate counselor reliance upon these considerations and in some cases lead to counseling that is even more directive.
Some women in the near future will face the choice of whether to abort, keep, or treat a genetically anomalous fetus.
Future counselors will have to figure out under what circumstances it is even appropriate to broach the subject. Should they only discuss therapies that are FDA-approved, or should they mention experimental treatments? What about interventions that are available in Europe or Asia, but banned in the United States? Or even in the best case of scenario of an FDA-approved treatment, should a counselor make reference to it if she knows for a fact that her client cannot possibly afford it?
Beyond the basic question of what information to share, counselors will have to confront the fact that the very notion of fixing or "editing" offspring will be repugnant to many women, and inherent in the suggestion is the stigmatization of individuals with disabilities. Prenatal genetic counselors will be on the forefront of debates surrounding which fetuses should remain as they are and which ones should be altered.
Despite these concerns, some women in the near future will face the choice of whether to abort, keep, or treat a genetically anomalous fetus in utero. Take, for example, a woman who learns during prenatal testing that her fetus has Angelman syndrome, a genetic disorder characterized by intellectual disability, speech impairment, loss of muscle control, epilepsy, and a small head. There is currently no human treatment for Angelman syndrome, which is caused by a loss of function in a single gene, UBE3A.
But scientists at the University of North Carolina have been able to treat Angelman syndrome in fetal mice by reactivating UBE3A through use of a single injection. The therapy has also proven effective in cultured human brain cells. This suggests that a woman might soon have to consider injecting her fetus's brain with a CRISPR concoction custom-designed to target UBE3A, rather than terminate her pregnancy or bring her fetus to term unaltered.
Assuming she receives the adequate information to make an informed choice, she too will face an ethical conundrum. There will be the inherent risks of injecting anything into a developing fetus's brain, including the possibility of infection, brain damage, and miscarriage. But there are also risks specific to gene editing, such as so-called off-target effects, the possibility of impacting genes other than the intended one. Such effects are highly unpredictable and can be difficult to detect. So too is it impossible to predict how altering UBE3A might lead to other genetic and epigenetic changes once the baby is born.
There are no easy answers to the many questions that will arise in this space.
A woman deciding how to act in this scenario must balance these risks against the potential benefits of the therapy, layered on top of her belief system, resources, and personal ethics. The calculus will be different for every woman, and even the same woman might change her mind from one pregnancy to the next based on the severity of the condition diagnosed and other available medical options.
Her genetic counselor, meanwhile, must be sensitive to all of these concerns in helping her make her decision, keeping up to date on the possible new treatments, and carefully choosing which information to disclose in striving to be neutral. There are no easy answers to the many questions that will arise in this space, but better to start thinking about them now, before it is too late.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
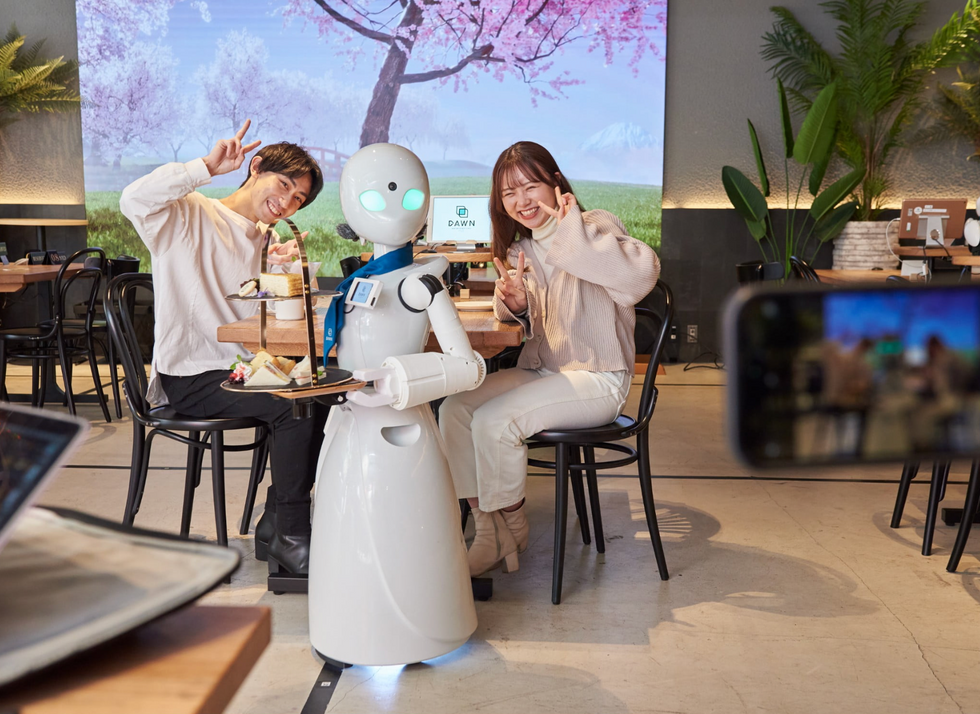
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
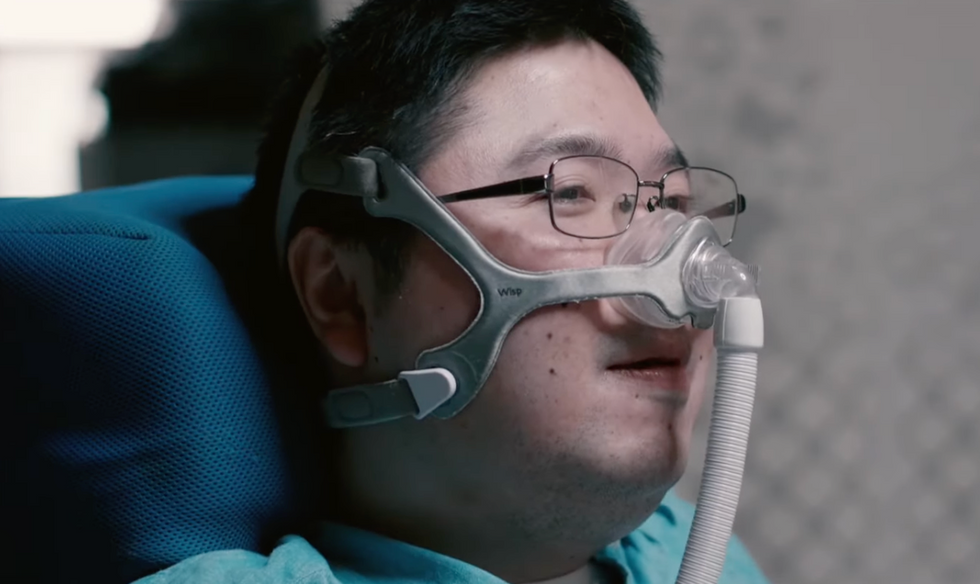
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

