For Kids with Progeria, New Therapies May Offer Revolutionary Hope for a Longer Life
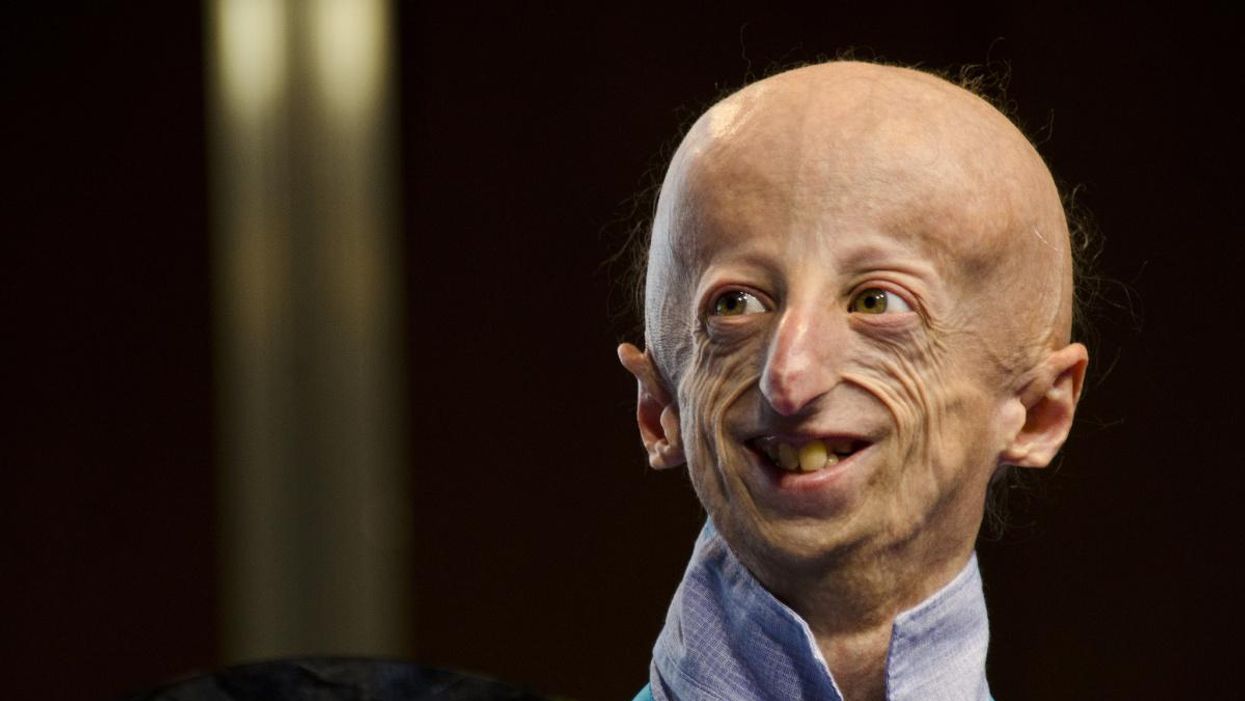
Sammy Basso is one of around 400 young people in the world with progeria.
Sammy Basso has some profound ideas about fate. As long as he has been alive, he has known he has minimal control over his own. His parents, however, had to transition from a world of unlimited possibility to one in which their son might not live to his 20s.
"I remember very clearly that day because Sammy was three years old," his mother says of the day a genetic counselor diagnosed Sammy with progeria. "It was a devastating day for me."
But to Sammy, he has always been himself: a smart kid, interested in science, a little smaller than his classmates, with one notable kink in his DNA. In one copy of the gene that codes for the protein Lamin A, Sammy has a T where there should be a C. The incorrect code creates a toxic protein called progerin, which destabilizes Sammy's cells and makes him age much faster than a person who doesn't have the mutation. The older he gets, the more he is in danger of strokes, heart failure, or a heart attack. "I am okay with my situation," he says from his home in Tezze sul Brenta, Italy. "But I think, yes, fate has a great role in my life."
Just 400 or so people in the world live with progeria: The mutation that causes it usually arises de novo, or "of new," meaning that it is not inherited but happens spontaneously during gestation. The challenge, as with all rare diseases, is that few cases means few treatments.
"When we first started, there was absolutely nothing out there," says Leslie Gordon, a physician-researcher who co-founded the Progeria Research Foundation in 1999 after her own son, also named Sam, was diagnosed with the disease. "We knew we had to jumpstart the entire field, so we collected money through road races and special events and writing grants and all sorts of donors… I think the first year we raised $75,000, most of it from one donor."
"We have not only the possibility but the responsibility to make the world a better world, and also to make a body a better body."
By 2003, the foundation had collaborated with Francis Collins, a geneticist who is now director of the National Institutes of Health, to work out the genetic basis for progeria—that single mutation Sammy has. The discovery led to interest in lonafarnib, a drug that was already being used in cancer patients but could potentially operate downstream of the mutation, preventing the buildup of the defective progerin in the body. "We funded cellular studies to look at a lonafarnib in cells, mouse studies to look at lonafarnib in mouse models of progeria… and then we initiated the clinical trials," Gordon says.
Sammy Basso's family had gotten involved with the Progeria Research Foundation through their international patient registry, which maintains relationships with families in 49 countries. "We started to hear about lonafarnib in 2006 from Leslie Gordon," says Sammy's father, Amerigo Basso, with his son translating. "She told us about the lonafarnib. And we were very happy because for the first time we understood that there was something that could help our son and our lives." Amerigo used the Italian word speranza, which means hope.
Still, Sammy wasn't sure if lonafarnib was right for him. "Since when I was very young I thought that everything happens for a reason. So, in my mind, if God made me with progeria, there was a reason, and to try to heal from progeria was something wrong," he says. Gradually, his parents and doctors, and Leslie Gordon, convinced him otherwise. Sammy began to believe that God was also the force behind doctors, science, and research. "And so we have not only the possibility but the responsibility to make the world a better world, and also to make a body a better body," he says.

Sammy Basso and his parents.
Courtesy of Basso
Sammy began taking lonafarnib, with the Progeria Research Foundation intermittently flying him, and other international trial participants, to Boston for tests. He was immediately beset by some of the drug's more unpleasant side effects: Stomach problems, nausea, and vomiting. "The first period was absolutely the worst period of my life," he says.
At first, doctors prescribed other medicines for the side effects, but to Sammy it had as much effect as drinking water. He visited doctor after doctor, with some calling him weekly or even daily to ask how he was doing. Eventually the specialists decided that he should lower his dose, balancing his pain with the benefit of the drug. Sammy can't actually feel any positive effect of the lonafarnib, but his health measurements have improved relative to people with progeria who don't take it.
While they never completely disappeared, Sammy's side effects decreased to the point that he could live. Inspired by the research that led to lonafarnib, he went to university to study molecular biology. For his thesis work, he travelled to Spain to perform experiments on cells and on mice with progeria, learning how to use the gene-editing technique CRISPR-Cas9 to cut out the mutated bit of DNA. "I was so excited to participate in this study," Sammy says. He felt like his work could make a difference.
In 2018, the Progeria Research Foundation was hosting one of their biennial workshops when Francis Collins, the researcher who had located the mutation behind progeria 15 years earlier, got in touch with Leslie Gordon. "Francis called me and said, Hey, I just saw a talk by David Liu from the Broad [Institute]. And it was pretty amazing. He has been looking at progeria and has very early, but very exciting data… Do you have any spaces, any slots you could make in your program for late breaking news?"
Gordon found a spot, and David Liu came to talk about what was going on in his lab, which was an even more advanced treatment that led to mice with the progeria mutation living into their senior mouse years—substantially closer to a normal lifespan. Liu's lab had built on the idea of CRISPR-Cas9 to create a more elegant genetic process called base editing: Instead of chopping out mutated DNA, a scientist could chemically convert an incorrect DNA letter to the correct one, like the search and replace function in word processing software. Mice who had their Lamin-A mutations corrected this way lived more than twice as long as untreated animals.
Sammy was in the audience at Dr. Liu's talk. "When I heard about this base editing as a younger scientist, I thought that I was living in the future," he says. "When my parents had my diagnosis of progeria, the science knew very little information about DNA. And now we are talking about healing the DNA… It is incredible."
Lonafarnib (also called Zokinvy) was approved by the US Food and Drug Administration this past November. Sammy, now 25, still takes it, and still manages his side effects. With luck, the gift of a few extra years will act as a bridge until he can try Liu's revolutionary new gene treatment, which has not yet begun testing in humans. While Leslie Gordon warns that she's always wrong about things like this, she hopes to see the new base editing techniques in clinical trials in the next year or two. Sammy won't need to be convinced to try it this time; his thinking on fate has evolved since his first encounter with lonafarnib.
"I would be very happy to try it," he says. "I know that for a non-scientist it can be difficult to understand. Some people think that we are the DNA. We are not. The DNA is a part of us, and to correct it is to do what we are already doing—just better." In short, a gene therapy, while it may seem like science fiction, is no different from a pill. For Sammy, both are a new way to think about fate: No longer something that simply happens to him.
A new type of cancer therapy is shrinking deadly brain tumors with just one treatment
MRI scans after a new kind of immunotherapy for brain cancer show remarkable progress in one patient just days after the first treatment.
Few cancers are deadlier than glioblastomas—aggressive and lethal tumors that originate in the brain or spinal cord. Five years after diagnosis, less than five percent of glioblastoma patients are still alive—and more often, glioblastoma patients live just 14 months on average after receiving a diagnosis.
But an ongoing clinical trial at Mass General Cancer Center is giving new hope to glioblastoma patients and their families. The trial, called INCIPIENT, is meant to evaluate the effects of a special type of immune cell, called CAR-T cells, on patients with recurrent glioblastoma.
How CAR-T cell therapy works
CAR-T cell therapy is a type of cancer treatment called immunotherapy, where doctors modify a patient’s own immune system specifically to find and destroy cancer cells. In CAR-T cell therapy, doctors extract the patient’s T-cells, which are immune system cells that help fight off disease—particularly cancer. These T-cells are harvested from the patient and then genetically modified in a lab to produce proteins on their surface called chimeric antigen receptors (thus becoming CAR-T cells), which makes them able to bind to a specific protein on the patient’s cancer cells. Once modified, these CAR-T cells are grown in the lab for several weeks so that they can multiply into an army of millions. When enough cells have been grown, these super-charged T-cells are infused back into the patient where they can then seek out cancer cells, bind to them, and destroy them. CAR-T cell therapies have been approved by the US Food and Drug Administration (FDA) to treat certain types of lymphomas and leukemias, as well as multiple myeloma, but haven’t been approved to treat glioblastomas—yet.
CAR-T cell therapies don’t always work against solid tumors, such as glioblastomas. Because solid tumors contain different kinds of cancer cells, some cells can evade the immune system’s detection even after CAR-T cell therapy, according to a press release from Massachusetts General Hospital. For the INCIPIENT trial, researchers modified the CAR-T cells even further in hopes of making them more effective against solid tumors. These second-generation CAR-T cells (called CARv3-TEAM-E T cells) contain special antibodies that attack EFGR, a protein expressed in the majority of glioblastoma tumors. Unlike other CAR-T cell therapies, these particular CAR-T cells were designed to be directly injected into the patient’s brain.
The INCIPIENT trial results
The INCIPIENT trial involved three patients who were enrolled in the study between March and July 2023. All three patients—a 72-year-old man, a 74-year-old man, and a 57-year-old woman—were treated with chemo and radiation and enrolled in the trial with CAR-T cells after their glioblastoma tumors came back.
The results, which were published earlier this year in the New England Journal of Medicine (NEJM), were called “rapid” and “dramatic” by doctors involved in the trial. After just a single infusion of the CAR-T cells, each patient experienced a significant reduction in their tumor sizes. Just two days after receiving the infusion, the glioblastoma tumor of the 72-year-old man decreased by nearly twenty percent. Just two months later the tumor had shrunk by an astonishing 60 percent, and the change was maintained for more than six months. The most dramatic result was in the 57-year-old female patient, whose tumor shrank nearly completely after just one infusion of the CAR-T cells.
The results of the INCIPIENT trial were unexpected and astonishing—but unfortunately, they were also temporary. For all three patients, the tumors eventually began to grow back regardless of the CAR-T cell infusions. According to the press release from MGH, the medical team is now considering treating each patient with multiple infusions or prefacing each treatment with chemotherapy to prolong the response.
While there is still “more to do,” says co-author of the study neuro-oncologist Dr. Elizabeth Gerstner, the results are still promising. If nothing else, these second-generation CAR-T cell infusions may someday be able to give patients more time than traditional treatments would allow.
“These results are exciting but they are also just the beginning,” says Dr. Marcela Maus, a doctor and professor of medicine at Mass General who was involved in the clinical trial. “They tell us that we are on the right track in pursuing a therapy that has the potential to change the outlook for this intractable disease.”
A recent study in The Lancet Oncology showed that AI found 20 percent more cancers on mammogram screens than radiologists alone.
Since the early 2000s, AI systems have eliminated more than 1.7 million jobs, and that number will only increase as AI improves. Some research estimates that by 2025, AI will eliminate more than 85 million jobs.
But for all the talk about job security, AI is also proving to be a powerful tool in healthcare—specifically, cancer detection. One recently published study has shown that, remarkably, artificial intelligence was able to detect 20 percent more cancers in imaging scans than radiologists alone.
Published in The Lancet Oncology, the study analyzed the scans of 80,000 Swedish women with a moderate hereditary risk of breast cancer who had undergone a mammogram between April 2021 and July 2022. Half of these scans were read by AI and then a radiologist to double-check the findings. The second group of scans was read by two researchers without the help of AI. (Currently, the standard of care across Europe is to have two radiologists analyze a scan before diagnosing a patient with breast cancer.)
The study showed that the AI group detected cancer in 6 out of every 1,000 scans, while the radiologists detected cancer in 5 per 1,000 scans. In other words, AI found 20 percent more cancers than the highly-trained radiologists.

But even though the AI was better able to pinpoint cancer on an image, it doesn’t mean radiologists will soon be out of a job. Dr. Laura Heacock, a breast radiologist at NYU, said in an interview with CNN that radiologists do much more than simply screening mammograms, and that even well-trained technology can make errors. “These tools work best when paired with highly-trained radiologists who make the final call on your mammogram. Think of it as a tool like a stethoscope for a cardiologist.”
AI is still an emerging technology, but more and more doctors are using them to detect different cancers. For example, researchers at MIT have developed a program called MIRAI, which looks at patterns in patient mammograms across a series of scans and uses an algorithm to model a patient's risk of developing breast cancer over time. The program was "trained" with more than 200,000 breast imaging scans from Massachusetts General Hospital and has been tested on over 100,000 women in different hospitals across the world. According to MIT, MIRAI "has been shown to be more accurate in predicting the risk for developing breast cancer in the short term (over a 3-year period) compared to traditional tools." It has also been able to detect breast cancer up to five years before a patient receives a diagnosis.
The challenges for cancer-detecting AI tools now is not just accuracy. AI tools are also being challenged to perform consistently well across different ages, races, and breast density profiles, particularly given the increased risks that different women face. For example, Black women are 42 percent more likely than white women to die from breast cancer, despite having nearly the same rates of breast cancer as white women. Recently, an FDA-approved AI device for screening breast cancer has come under fire for wrongly detecting cancer in Black patients significantly more often than white patients.
As AI technology improves, radiologists will be able to accurately scan a more diverse set of patients at a larger volume than ever before, potentially saving more lives than ever.

