With this new technology, hospitals and pharmacies could make vaccines and medicines onsite
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.

New research focuses on methods that could change medicine-making worldwide. The scientists propose bursting cells open, removing their DNA and using the cellular gears inside to make therapies.
Most modern biopharmaceutical medicines are produced by workhorse cells—typically bacterial but sometimes mammalian. The cells receive the synthesizing instructions on a snippet of a genetic code, which they incorporate into their DNA. The cellular machinery—ribosomes, RNAs, polymerases, and other compounds—read and use these instructions to build the medicinal molecules, which are harvested and administered to patients.
Although a staple of modern pharma, this process is complex and expensive. One must first insert the DNA instructions into the cells, which they may or may not uptake. One then must grow the cells, keeping them alive and well, so that they produce the required therapeutics, which then must be isolated and purified. To make this at scale requires massive bioreactors and big factories from where the drugs are distributed—and may take a while to arrive where they’re needed. “The pandemic showed us that this method is slow and cumbersome,” says Govind Rao, professor of biochemical engineering who directs the Center for Advanced Sensor Technology at the University of Maryland, Baltimore County (UMBC). “We need better methods that can work faster and can work locally where an outbreak is happening.”
Rao and his team of collaborators, which spans multiple research institutions, believe they have a better approach that may change medicine-making worldwide. They suggest forgoing the concept of using living cells as medicine-producers. Instead, they propose breaking the cells and using the remaining cellular gears for assembling the therapeutic compounds. Instead of inserting the DNA into living cells, the team burst them open, and removed their DNA altogether. Yet, the residual molecular machinery of ribosomes, polymerases and other cogwheels still functioned the way it would in a cell. “Now if you drop your DNA drug-making instructions into that soup, this machinery starts making what you need,” Rao explains. “And because you're no longer worrying about living cells, it becomes much simpler and more efficient.” The collaborators detail their cell-free protein synthesis or CFPS method in their recent paper published in preprint BioAxiv.
While CFPS does not use living cells, it still needs the basic building blocks to assemble proteins from—such as amino acids, nucleotides and certain types of enzymes. These are regularly added into this “soup” to keep the molecular factory chugging. “We just mix everything in as a batch and we let it integrate,” says James Robert Swartz, professor of chemical engineering and bioengineering at Stanford University and co-author of the paper. “And we make sure that we provide enough oxygen.” Rao likens the process to making milk from milk powder.
For a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology.
The idea of a cell-free protein synthesis is older than one might think. Swartz first experimented with it around 1997, when he was a chemical engineer at Genentech. While working on engineering bacteria to make pharmaceuticals, he discovered that there was a limit to what E. coli cells, the workhorse darling of pharma, could do. For example, it couldn’t grow and properly fold some complex proteins. “We tried many genetic engineering approaches, many fermentation, development, and environmental control approaches,” Swartz recalls—to no avail.
“The organism had its own agenda,” he quips. “And because everything was happening within the organism, we just couldn't really change those conditions very easily. Some of them we couldn’t change at all—we didn’t have control.”
It was out of frustration with the defiant bacteria that a new idea took hold. Could the cells be opened instead, so that the protein-forming reactions could be influenced more easily? “Obviously, we’d lose the ability for them to reproduce,” Swartz says. But that also meant that they no longer needed to keep the cells alive and could focus on making the specific reactions happen. “We could take the catalysts, the enzymes, and the more complex catalysts and activate them, make them work together, much as they would in a living cell, but the way we wanted.”
In 1998, Swartz joined Stanford, and began perfecting the biochemistry of the cell-free method, identifying the reactions he wanted to foster and stopping those he didn’t want. He managed to make the idea work, but for a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology. For their BioArxiv paper, the team tested the method by growing a specific antiviral protein called griffithsin.
First identified by Barry O’Keefe at National Cancer Institute over a decade ago, griffithsin is an antiviral known to interfere with many viruses’ ability to enter cells—including HIV, SARS, SARS-CoV-2, MERS and others. Originally isolated from the red algae Griffithsia, it works differently from antibodies and antibody cocktails.
Most antiviral medicines tend to target the specific receptors that viruses use to gain entry to the cells they infect. For example, SARS-CoV-2 uses the infamous spike protein to latch onto the ACE2 receptor of mammalian cells. The antibodies or other antiviral molecules stick to the spike protein, shutting off its ability to cling onto the ACE2 receptors. Unfortunately, the spike proteins mutate very often, so the medicines lose their potency. On the contrary, griffithsin has the ability to cling to the different parts of viral shells called capsids—namely to the molecules of mannose, a type of sugar. That extra stuff, glued all around the capsid like dead weight, makes it impossible for the virus to squeeze into the cell.
“Every time we have a vaccine or an antibody against a specific SARS-CoV-2 strain, that strain then mutates and so you lose efficacy,” Rao explains. “But griffithsin molecules glom onto the viral capsid, so the capsid essentially becomes a sticky mess and can’t enter the cell.” Mannose molecules also don’t mutate as easily as viruses’ receptors, so griffithsin-based antivirals do not have to be constantly updated. And because mannose molecules are found on many viruses’ capsids, it makes griffithsin “a universal neutralizer,” Rao explains.
“When griffithsin was discovered, we recognized that it held a lot of promise as a potential antiviral agent,” O’Keefe says. In 2010, he published a paper about griffithsin efficacy in neutralizing viruses of the corona family—after the first SARS outbreak in the early 2000s, the scientific community was interested in such antivirals. Yet, griffithsin is still not available as an off-the-shelf product. So during the Covid pandemic, the team experimented with synthesizing griffithsin using the cell-free production method. They were able to generate potent griffithsin in less than 24 hours without having to grow living cells.
The antiviral protein isn't the only type of medicine that can be made cell-free. The proteins needed for vaccine production could also be made the same way. “Such portable, on-demand drug manufacturing platforms can produce antiviral proteins within hours, making them ideal for combating future pandemics,” Rao says. “We would be able to stop the pandemic before it spreads.”
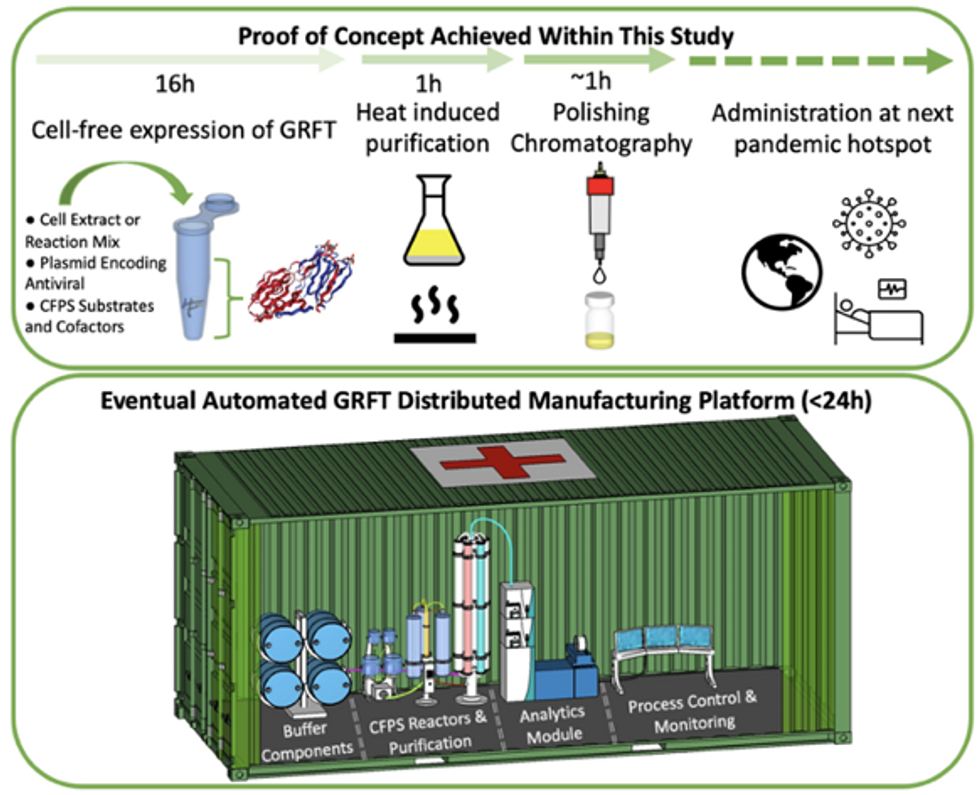
Top: Describes the process used in the study. Bottom: Describes how the new medicines and vaccines could be made at the site of a future viral outbreak.
Image courtesy of Rao and team, sourced from An approach to rapid distributed manufacturing of broad spectrumanti-viral griffithsin using cell-free systems to mitigate pandemics.
Rao’s idea is to perfect the technology to the point that any hospital or pharmacy can load up the media containing molecular factories, mix up the required amino acids, nucleotides and enzymes, and harvest the meds within hours. That will allow making medicines onsite and on demand. “That would be a self-contained production unit, so that you could just ship the production wherever the pandemic is breaking out,” says Swartz.
These units and the meds they produce, will, of course, have to undergo rigorous testing. “The biggest hurdles will be validating these against conventional technology,” Rao says. The biotech industry is risk-averse and prefers the familiar methods. But if this approach works, it may go beyond emergency situations and revolutionize the medicine-making paradigm even outside hospitals and pharmacies. Rao hopes that someday the method might become so mainstream that people may be able to buy and operate such reactors at home. “You can imagine a diabetic patient making insulin that way, or some other drugs,” Rao says. It would work not unlike making baby formula from the mere white powder. Just add water—and some oxygen, too.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
Is there a robot nanny in your child's future?
Some researchers argue that active, playful engagement with a "robot nanny" for a few hours a day is better than several hours in front of a TV or with an iPad.
From ROBOTS AND THE PEOPLE WHO LOVE THEM: Holding on to Our Humanity in an Age of Social Robots by Eve Herold. Copyright © 2024 by the author and reprinted by permission of St. Martin’s Publishing Group.
Could the use of robots take some of the workload off teachers, add engagement among students, and ultimately invigorate learning by taking it to a new level that is more consonant with the everyday experiences of young people? Do robots have the potential to become full-fledged educators and further push human teachers out of the profession? The preponderance of opinion on this subject is that, just as AI and medical technology are not going to eliminate doctors, robot teachers will never replace human teachers. Rather, they will change the job of teaching.
A 2017 study led by Google executive James Manyika suggested that skills like creativity, emotional intelligence, and communication will always be needed in the classroom and that robots aren’t likely to provide them at the same level that humans naturally do. But robot teachers do bring advantages, such as a depth of subject knowledge that teachers can’t match, and they’re great for student engagement.
The teacher and robot can complement each other in new ways, with the teacher facilitating interactions between robots and students. So far, this is the case with teaching “assistants” being adopted now in China, Japan, the U.S., and Europe. In this scenario, the robot (usually the SoftBank child-size robot NAO) is a tool for teaching mainly science, technology, engineering, and math (the STEM subjects), but the teacher is very involved in planning, overseeing, and evaluating progress. The students get an entertaining and enriched learning experience, and some of the teaching load is taken off the teacher. At least, that’s what researchers have been able to observe so far.
To be sure, there are some powerful arguments for having robots in the classroom. A not-to-be-underestimated one is that robots “speak the language” of today’s children, who have been steeped in technology since birth. These children are adept at navigating a media-rich environment that is highly visual and interactive. They are plugged into the Internet 24-7. They consume music, games, and huge numbers of videos on a weekly basis. They expect to be dazzled because they are used to being dazzled by more and more spectacular displays of digital artistry. Education has to compete with social media and the entertainment vehicles of students’ everyday lives.
Another compelling argument for teaching robots is that they help prepare students for the technological realities they will encounter in the real world when robots will be ubiquitous. From childhood on, they will be interacting and collaborating with robots in every sphere of their lives from the jobs they do to dealing with retail robots and helper robots in the home. Including robots in the classroom is one way of making sure that children of all socioeconomic backgrounds will be better prepared for a highly automated age, when successfully using robots will be as essential as reading and writing. We’ve already crossed this threshold with computers and smartphones.
Students need multimedia entertainment with their teaching. This is something robots can provide through their ability to connect to the Internet and act as a centralized host to videos, music, and games. Children also need interaction, something robots can deliver up to a point, but which humans can surpass. The education of a child is not just intended to make them technologically functional in a wired world, it’s to help them grow in intellectual, creative, social, and emotional ways. When considered through this perspective, it opens the door to questions concerning just how far robots should go. Robots don’t just teach and engage children; they’re designed to tug at their heartstrings.
It’s no coincidence that many toy makers and manufacturers are designing cute robots that look and behave like real children or animals, says Turkle. “When they make eye contact and gesture toward us, they predispose us to view them as thinking and caring,” she has written in The Washington Post. “They are designed to be cute, to provide a nurturing response” from the child. As mentioned previously, this nurturing experience is a powerful vehicle for drawing children in and promoting strong attachment. But should children really love their robots?

ROBOTS AND THE PEOPLE WHO LOVE THEM: Holding on to Our Humanity in an Age of Social Robots by Eve Herold (January 9, 2024).
St. Martin’s Publishing Group
The problem, once again, is that a child can be lulled into thinking that she’s in an actual relationship, when a robot can’t possibly love her back. If adults have these vulnerabilities, what might such asymmetrical relationships do to the emotional development of a small child? Turkle notes that while we tend to ascribe a mind and emotions to a socially interactive robot, “simulated thinking may be thinking, but simulated feeling is never feeling, and simulated love is never love.”
Always a consideration is the fact that in the first few years of life, a child’s brain is undergoing rapid growth and development that will form the foundation of their lifelong emotional health. These formative experiences are literally shaping the child’s brain, their expectations, and their view of the world and their place in it. In Alone Together, Turkle asks: What are we saying to children about their importance to us when we’re willing to outsource their care to a robot? A child might be superficially entertained by the robot while his self-esteem is systematically undermined.
Research has emerged showing that there are clear downsides to child-robot relationships.
Still, in the case of robot nannies in the home, is active, playful engagement with a robot for a few hours a day any more harmful than several hours in front of a TV or with an iPad? Some, like Xiong, regard interacting with a robot as better than mere passive entertainment. iPal’s manufacturers say that their robot can’t replace parents or teachers and is best used by three- to eight-year-olds after school, while they wait for their parents to get off work. But as robots become ever-more sophisticated, they’re expected to perform more of the tasks of day-to-day care and to be much more emotionally advanced. There is no question children will form deep attachments to some of them. And research has emerged showing that there are clear downsides to child-robot relationships.
Some studies, performed by Turkle and fellow MIT colleague Cynthia Breazeal, have revealed a darker side to the child-robot bond. Turkle has reported extensively on these studies in The Washington Post and in her book Alone Together. Most children love robots, but some act out their inner bully on the hapless machines, hitting and kicking them and otherwise trying to hurt them. The trouble is that the robot can’t fight back, teaching children that they can bully and abuse without consequences. As in any other robot relationship, such harmful behavior could carry over into the child’s human relationships.
And, ironically, it turns out that communicative machines don’t actually teach kids good communication skills. It’s well known that parent-child communication in the first three years of life sets the stage for a very young child’s intellectual and academic success. Verbal back-and-forth with parents and care-givers is like fuel for a child’s growing brain. One article that examined several types of play and their effect on children’s communication skills, published in JAMA Pediatrics in 2015, showed that babies who played with electronic toys—like the popular robot dog Aibo—show a decrease in both the quantity and quality of their language skills.
Anna V. Sosa of the Child Speech and Language Lab at Northern Arizona University studied twenty-six ten- to sixteen- month-old infants to compare the growth of their language skills after they played with three types of toys: electronic toys like a baby laptop and talking farm; traditional toys like wooden puzzles and building blocks; and books read aloud by their parents. The play that produced the most growth in verbal ability was having books read to them by a caregiver, followed by play with traditional toys. Language gains after playing with electronic toys came dead last. This form of play involved the least use of adult words, the least conversational turntaking, and the least verbalizations from the children. While the study sample was small, it’s not hard to extrapolate that no electronic toy or even more abled robot could supply the intimate responsiveness of a parent reading stories to a child, explaining new words, answering the child’s questions, and modeling the kind of back- and-forth interaction that promotes empathy and reciprocity in relationships.
***
Most experts acknowledge that robots can be valuable educational tools. But they can’t make a child feel truly loved, validated, and valued. That’s the job of parents, and when parents abdicate this responsibility, it’s not only the child who misses out on one of life’s most profound experiences.
We really don’t know how the tech-savvy children of today will ultimately process their attachments to robots and whether they will be excessively predisposed to choosing robot companionship over that of humans. It’s possible their techno literacy will draw for them a bold line between real life and a quasi-imaginary history with a robot. But it will be decades before we see long-term studies culminating in sufficient data to help scientists, and the rest of us, to parse out the effects of a lifetime spent with robots.
This is an excerpt from ROBOTS AND THE PEOPLE WHO LOVE THEM: Holding on to Our Humanity in an Age of Social Robots by Eve Herold. The book will be published on January 9, 2024.
Stem cells from a fetus can travel to the heart and regenerate the muscle, essentially saving a mother’s life.
Story by Big Think
In rare cases, a woman’s heart can start to fail in the months before or after giving birth. The all-important muscle weakens as its chambers enlarge, reducing the amount of blood pumped with each beat. Peripartum cardiomyopathy can threaten the lives of both mother and child. Viral illness, nutritional deficiency, the bodily stress of pregnancy, or an abnormal immune response could all play a role, but the causes aren’t concretely known.
If there is a silver lining to peripartum cardiomyopathy, it’s that it is perhaps the most survivable form of heart failure. A remarkable 50% of women recover spontaneously. And there’s an even more remarkable explanation for that glowing statistic: The fetus‘ stem cells migrate to the heart and regenerate the beleaguered muscle. In essence, the developing or recently born child saves its mother’s life.
Saving mama
While this process has not been observed directly in humans, it has been witnessed in mice. In a 2015 study, researchers tracked stem cells from fetal mice as they traveled to mothers’ damaged cardiac cells and integrated themselves into hearts.
Evolutionarily, this function makes sense: It is in the fetus’ best interest that its mother remains healthy.
Scientists also have spotted cells from the fetus within the hearts of human mothers, as well as countless other places inside the body, including the skin, spleen, liver, brain, lung, kidney, thyroid, lymph nodes, salivary glands, gallbladder, and intestine. These cells essentially get everywhere. While most are eliminated by the immune system during pregnancy, some can persist for an incredibly long time — up to three decades after childbirth.
This integration of the fetus’ cells into the mother’s body has been given a name: fetal microchimerism. The process appears to start between the fourth and sixth week of gestation in humans. Scientists are actively trying to suss out its purpose. Fetal stem cells, which can differentiate into all sorts of specialized cells, appear to target areas of injury. So their role in healing seems apparent. Evolutionarily, this function makes sense: It is in the fetus’ best interest that its mother remains healthy.
Sending cells into the mother’s body may also prime her immune system to grow more tolerant of the developing fetus. Successful pregnancy requires that the immune system not see the fetus as an interloper and thus dispatch cells to attack it.
Fetal microchimerism
But fetal microchimerism might not be entirely beneficial. Greater concentrations of the cells have been associated with various autoimmune diseases such as lupus, Sjogren’s syndrome, and even multiple sclerosis. After all, they are foreign cells living in the mother’s body, so it’s possible that they might trigger subtle, yet constant inflammation. Fetal cells also have been linked to cancer, although it isn’t clear whether they abet or hinder the disease.
A team of Spanish scientists summarized the apparent give and take of fetal microchimerism in a 2022 review article. “On the one hand, fetal microchimerism could be a source of progenitor cells with a beneficial effect on the mother’s health by intervening in tissue repair, angiogenesis, or neurogenesis. On the other hand, fetal microchimerism might have a detrimental function by activating the immune response and contributing to autoimmune diseases,” they wrote.
Regardless of a fetus’ cells net effect, their existence alone is intriguing. In a paper published earlier this year, University of London biologist Francisco Úbeda and University of Western Ontario mathematical biologist Geoff Wild noted that these cells might very well persist within mothers for life.
“Therefore, throughout their reproductive lives, mothers accumulate fetal cells from each of their past pregnancies including those resulting in miscarriages. Furthermore, mothers inherit, from their own mothers, a pool of cells contributed by all fetuses carried by their mothers, often referred to as grandmaternal microchimerism.”
So every mother may carry within her literal pieces of her ancestors.