With this new technology, hospitals and pharmacies could make vaccines and medicines onsite
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.

New research focuses on methods that could change medicine-making worldwide. The scientists propose bursting cells open, removing their DNA and using the cellular gears inside to make therapies.
Most modern biopharmaceutical medicines are produced by workhorse cells—typically bacterial but sometimes mammalian. The cells receive the synthesizing instructions on a snippet of a genetic code, which they incorporate into their DNA. The cellular machinery—ribosomes, RNAs, polymerases, and other compounds—read and use these instructions to build the medicinal molecules, which are harvested and administered to patients.
Although a staple of modern pharma, this process is complex and expensive. One must first insert the DNA instructions into the cells, which they may or may not uptake. One then must grow the cells, keeping them alive and well, so that they produce the required therapeutics, which then must be isolated and purified. To make this at scale requires massive bioreactors and big factories from where the drugs are distributed—and may take a while to arrive where they’re needed. “The pandemic showed us that this method is slow and cumbersome,” says Govind Rao, professor of biochemical engineering who directs the Center for Advanced Sensor Technology at the University of Maryland, Baltimore County (UMBC). “We need better methods that can work faster and can work locally where an outbreak is happening.”
Rao and his team of collaborators, which spans multiple research institutions, believe they have a better approach that may change medicine-making worldwide. They suggest forgoing the concept of using living cells as medicine-producers. Instead, they propose breaking the cells and using the remaining cellular gears for assembling the therapeutic compounds. Instead of inserting the DNA into living cells, the team burst them open, and removed their DNA altogether. Yet, the residual molecular machinery of ribosomes, polymerases and other cogwheels still functioned the way it would in a cell. “Now if you drop your DNA drug-making instructions into that soup, this machinery starts making what you need,” Rao explains. “And because you're no longer worrying about living cells, it becomes much simpler and more efficient.” The collaborators detail their cell-free protein synthesis or CFPS method in their recent paper published in preprint BioAxiv.
While CFPS does not use living cells, it still needs the basic building blocks to assemble proteins from—such as amino acids, nucleotides and certain types of enzymes. These are regularly added into this “soup” to keep the molecular factory chugging. “We just mix everything in as a batch and we let it integrate,” says James Robert Swartz, professor of chemical engineering and bioengineering at Stanford University and co-author of the paper. “And we make sure that we provide enough oxygen.” Rao likens the process to making milk from milk powder.
For a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology.
The idea of a cell-free protein synthesis is older than one might think. Swartz first experimented with it around 1997, when he was a chemical engineer at Genentech. While working on engineering bacteria to make pharmaceuticals, he discovered that there was a limit to what E. coli cells, the workhorse darling of pharma, could do. For example, it couldn’t grow and properly fold some complex proteins. “We tried many genetic engineering approaches, many fermentation, development, and environmental control approaches,” Swartz recalls—to no avail.
“The organism had its own agenda,” he quips. “And because everything was happening within the organism, we just couldn't really change those conditions very easily. Some of them we couldn’t change at all—we didn’t have control.”
It was out of frustration with the defiant bacteria that a new idea took hold. Could the cells be opened instead, so that the protein-forming reactions could be influenced more easily? “Obviously, we’d lose the ability for them to reproduce,” Swartz says. But that also meant that they no longer needed to keep the cells alive and could focus on making the specific reactions happen. “We could take the catalysts, the enzymes, and the more complex catalysts and activate them, make them work together, much as they would in a living cell, but the way we wanted.”
In 1998, Swartz joined Stanford, and began perfecting the biochemistry of the cell-free method, identifying the reactions he wanted to foster and stopping those he didn’t want. He managed to make the idea work, but for a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology. For their BioArxiv paper, the team tested the method by growing a specific antiviral protein called griffithsin.
First identified by Barry O’Keefe at National Cancer Institute over a decade ago, griffithsin is an antiviral known to interfere with many viruses’ ability to enter cells—including HIV, SARS, SARS-CoV-2, MERS and others. Originally isolated from the red algae Griffithsia, it works differently from antibodies and antibody cocktails.
Most antiviral medicines tend to target the specific receptors that viruses use to gain entry to the cells they infect. For example, SARS-CoV-2 uses the infamous spike protein to latch onto the ACE2 receptor of mammalian cells. The antibodies or other antiviral molecules stick to the spike protein, shutting off its ability to cling onto the ACE2 receptors. Unfortunately, the spike proteins mutate very often, so the medicines lose their potency. On the contrary, griffithsin has the ability to cling to the different parts of viral shells called capsids—namely to the molecules of mannose, a type of sugar. That extra stuff, glued all around the capsid like dead weight, makes it impossible for the virus to squeeze into the cell.
“Every time we have a vaccine or an antibody against a specific SARS-CoV-2 strain, that strain then mutates and so you lose efficacy,” Rao explains. “But griffithsin molecules glom onto the viral capsid, so the capsid essentially becomes a sticky mess and can’t enter the cell.” Mannose molecules also don’t mutate as easily as viruses’ receptors, so griffithsin-based antivirals do not have to be constantly updated. And because mannose molecules are found on many viruses’ capsids, it makes griffithsin “a universal neutralizer,” Rao explains.
“When griffithsin was discovered, we recognized that it held a lot of promise as a potential antiviral agent,” O’Keefe says. In 2010, he published a paper about griffithsin efficacy in neutralizing viruses of the corona family—after the first SARS outbreak in the early 2000s, the scientific community was interested in such antivirals. Yet, griffithsin is still not available as an off-the-shelf product. So during the Covid pandemic, the team experimented with synthesizing griffithsin using the cell-free production method. They were able to generate potent griffithsin in less than 24 hours without having to grow living cells.
The antiviral protein isn't the only type of medicine that can be made cell-free. The proteins needed for vaccine production could also be made the same way. “Such portable, on-demand drug manufacturing platforms can produce antiviral proteins within hours, making them ideal for combating future pandemics,” Rao says. “We would be able to stop the pandemic before it spreads.”
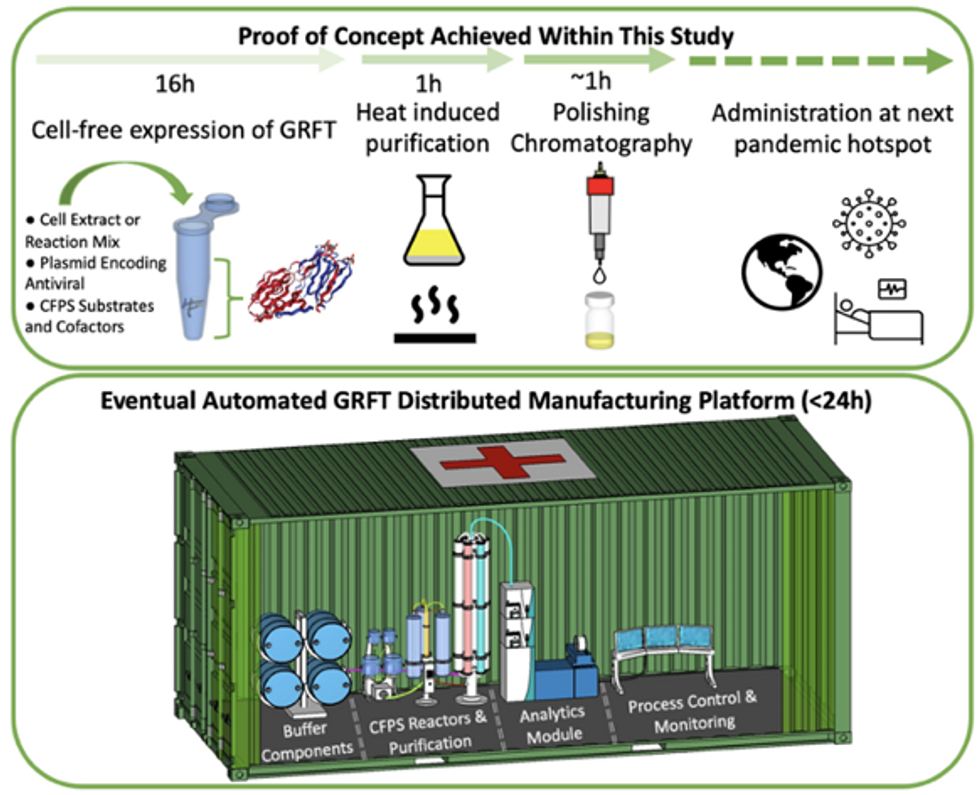
Top: Describes the process used in the study. Bottom: Describes how the new medicines and vaccines could be made at the site of a future viral outbreak.
Image courtesy of Rao and team, sourced from An approach to rapid distributed manufacturing of broad spectrumanti-viral griffithsin using cell-free systems to mitigate pandemics.
Rao’s idea is to perfect the technology to the point that any hospital or pharmacy can load up the media containing molecular factories, mix up the required amino acids, nucleotides and enzymes, and harvest the meds within hours. That will allow making medicines onsite and on demand. “That would be a self-contained production unit, so that you could just ship the production wherever the pandemic is breaking out,” says Swartz.
These units and the meds they produce, will, of course, have to undergo rigorous testing. “The biggest hurdles will be validating these against conventional technology,” Rao says. The biotech industry is risk-averse and prefers the familiar methods. But if this approach works, it may go beyond emergency situations and revolutionize the medicine-making paradigm even outside hospitals and pharmacies. Rao hopes that someday the method might become so mainstream that people may be able to buy and operate such reactors at home. “You can imagine a diabetic patient making insulin that way, or some other drugs,” Rao says. It would work not unlike making baby formula from the mere white powder. Just add water—and some oxygen, too.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
New approach to brain health is sparking memories
This fall, Robert Reinhart of Boston University published a study finding that electrical stimulation can boost memory - and Reinhart was surprised to discover the effects lasted a full month.
What if a few painless electrical zaps to your brain could help you recall names, perform better on Wordle or even ward off dementia?
This is where neuroscientists are going in efforts to stave off age-related memory loss as well as Alzheimer’s disease. Medications have shown limited effectiveness in reversing or managing loss of brain function so far. But new studies suggest that firing up an aging neural network with electrical or magnetic current might keep brains spry as we age.
Welcome to non-invasive brain stimulation (NIBS). No surgery or anesthesia is required. One day, a jolt in the morning with your own battery-operated kit could replace your wake-up coffee.
Scientists believe brain circuits tend to uncouple as we age. Since brain neurons communicate by exchanging electrical impulses with each other, the breakdown of these links and associations could be what causes the “senior moment”—when you can’t remember the name of the movie you just watched.
In 2019, Boston University researchers led by Robert Reinhart, director of the Cognitive and Clinical Neuroscience Laboratory, showed that memory loss in healthy older adults is likely caused by these disconnected brain networks. When Reinhart and his team stimulated two key areas of the brain with mild electrical current, they were able to bring the brains of older adult subjects back into sync — enough so that their ability to remember small differences between two images matched that of much younger subjects for at least 50 minutes after the testing stopped.
Reinhart wowed the neuroscience community once again this fall. His newer study in Nature Neuroscience presented 150 healthy participants, ages 65 to 88, who were able to recall more words on a given list after 20 minutes of low-intensity electrical stimulation sessions over four consecutive days. This amounted to a 50 to 65 percent boost in their recall.
Even Reinhart was surprised to discover the enhanced performance of his subjects lasted a full month when they were tested again later. Those who benefited most were the participants who were the most forgetful at the start.

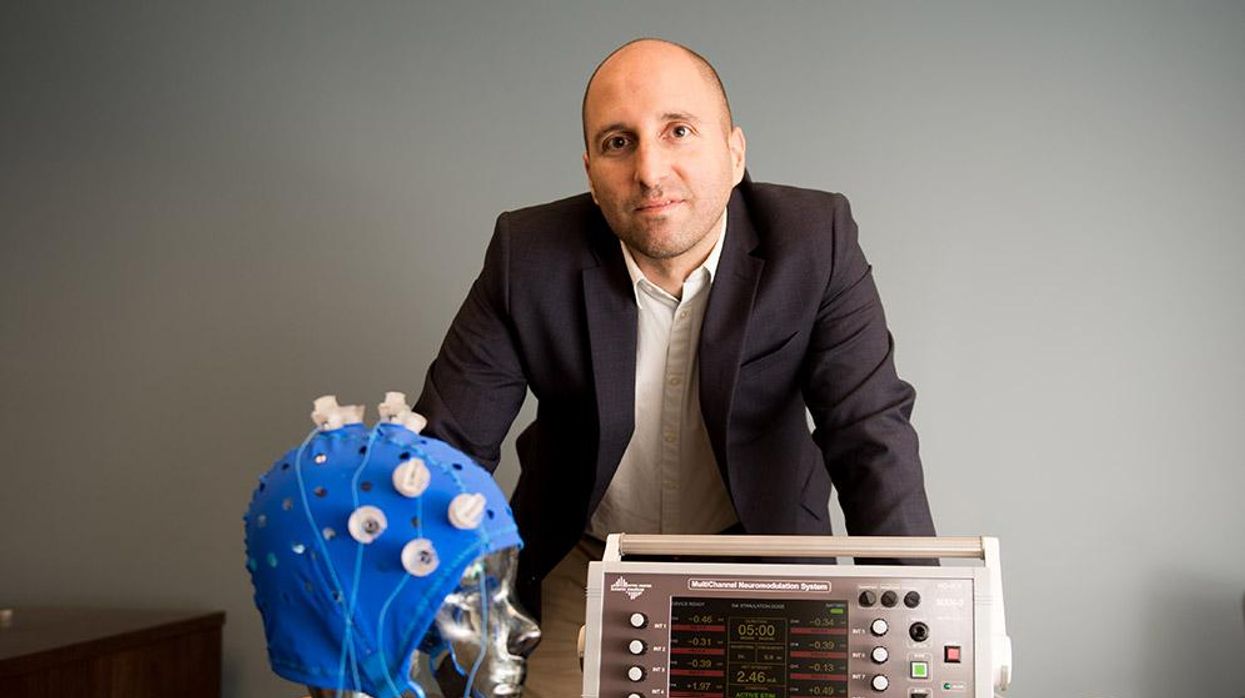
An older person participates in Robert Reinhart's research on brain stimulation.
Robert Reinhart
Reinhart’s subjects only suffered normal age-related memory deficits, but NIBS has great potential to help people with cognitive impairment and dementia, too, says Krista Lanctôt, the Bernick Chair of Geriatric Psychopharmacology at Sunnybrook Health Sciences Center in Toronto. Plus, “it is remarkably safe,” she says.
Lanctôt was the senior author on a meta-analysis of brain stimulation studies published last year on people with mild cognitive impairment or later stages of Alzheimer’s disease. The review concluded that magnetic stimulation to the brain significantly improved the research participants’ neuropsychiatric symptoms, such as apathy and depression. The stimulation also enhanced global cognition, which includes memory, attention, executive function and more.
This is the frontier of neuroscience.
The two main forms of NIBS – and many questions surrounding them
There are two types of NIBS. They differ based on whether electrical or magnetic stimulation is used to create the electric field, the type of device that delivers the electrical current and the strength of the current.
Transcranial Current Brain Stimulation (tES) is an umbrella term for a group of techniques using low-wattage electrical currents to manipulate activity in the brain. The current is delivered to the scalp or forehead via electrodes attached to a nylon elastic cap or rubber headband.
Variations include how the current is delivered—in an alternating pattern or in a constant, direct mode, for instance. Tweaking frequency, potency or target brain area can produce different effects as well. Reinhart’s 2022 study demonstrated that low or high frequencies and alternating currents were uniquely tied to either short-term or long-term memory improvements.
Sessions may be 20 minutes per day over the course of several days or two weeks. “[The subject] may feel a tingling, warming, poking or itching sensation,” says Reinhart, which typically goes away within a minute.
The other main approach to NIBS is Transcranial Magnetic Simulation (TMS). It involves the use of an electromagnetic coil that is held or placed against the forehead or scalp to activate nerve cells in the brain through short pulses. The stimulation is stronger than tES but similar to a magnetic resonance imaging (MRI) scan.
The subject may feel a slight knocking or tapping on the head during a 20-to-60-minute session. Scalp discomfort and headaches are reported by some; in very rare cases, a seizure can occur.
No head-to-head trials have been conducted yet to evaluate the differences and effectiveness between electrical and magnetic current stimulation, notes Lanctôt, who is also a professor of psychiatry and pharmacology at the University of Toronto. Although TMS was approved by the FDA in 2008 to treat major depression, both techniques are considered experimental for the purpose of cognitive enhancement.
“One attractive feature of tES is that it’s inexpensive—one-fifth the price of magnetic stimulation,” Reinhart notes.
Don’t confuse either of these procedures with the horrors of electroconvulsive therapy (ECT) in the 1950s and ‘60s. ECT is a more powerful, riskier procedure used only as a last resort in treating severe mental illness today.
Clinical studies on NIBS remain scarce. Standardized parameters and measures for testing have not been developed. The high heterogeneity among the many existing small NIBS studies makes it difficult to draw general conclusions. Few of the studies have been replicated and inconsistencies abound.
Scientists are still lacking so much fundamental knowledge about the brain and how it works, says Reinhart. “We don’t know how information is represented in the brain or how it’s carried forward in time. It’s more complex than physics.”
Lanctôt’s meta-analysis showed improvements in global cognition from delivering the magnetic form of the stimulation to people with Alzheimer’s, and this finding was replicated inan analysis in the Journal of Prevention of Alzheimer’s Disease this fall. Neither meta-analysis found clear evidence that applying the electrical currents, was helpful for Alzheimer’s subjects, but Lanctôt suggests this might be merely because the sample size for tES was smaller compared to the groups that received TMS.
At the same time, London neuroscientist Marco Sandrini, senior lecturer in psychology at the University of Roehampton, critically reviewed a series of studies on the effects of tES on episodic memory. Often declining with age, episodic memory relates to recalling a person’s own experiences from the past. Sandrini’s review concluded that delivering tES to the prefrontal or temporoparietal cortices of the brain might enhance episodic memory in older adults with Alzheimer’s disease and amnesiac mild cognitive impairment (the predementia phase of Alzheimer’s when people start to have symptoms).
Researchers readily tick off studies needed to explore, clarify and validate existing NIBS data. What is the optimal stimulus session frequency, spacing and duration? How intense should the stimulus be and where should it be targeted for what effect? How might genetics or degree of brain impairment affect responsiveness? Would adjunct medication or cognitive training boost positive results? Could administering the stimulus while someone sleeps expedite memory consolidation?
Using MRI or another brain scan along with computational modeling of the current flow, a clinician could create a treatment that is customized to each person’s brain.
While Sandrini’s review reported improvements induced by tES in the recall or recognition of words and images, there is no evidence it will translate into improvements in daily activities. This is another question that will require more research and testing, Sandrini notes.
Scientists are still lacking so much fundamental knowledge about the brain and how it works, says Reinhart. “We don’t know how information is represented in the brain or how it’s carried forward in time. It’s more complex than physics.”
Where the science is headed
Learning how to apply precision medicine to NIBS is the next focus in advancing this technology, says Shankar Tumati, a post-doctoral fellow working with Lanctôt.
There is great variability in each person’s brain anatomy—the thickness of the skull, the brain’s unique folds, the amount of cerebrospinal fluid. All of these structural differences impact how electrical or magnetic stimulation is distributed in the brain and ultimately the effects.
Using MRI or another brain scan along with computational modeling of the current flow, a clinician could create a treatment that is customized to each person’s brain, from where to put the electrodes to determining the exact dose and duration of stimulation needed to achieve lasting results, Sandrini says.
Above all, most neuroscientists say that largescale research studies over long periods of time are necessary to confirm the safety and durability of this therapy for the purpose of boosting memory. Short of that, there can be no FDA approval or medical regulation for this clinical use.
Lanctôt urges people to seek out clinical NIBS trials in their area if they want to see the science advance. “That is how we’ll find the answers,” she says, predicting it will be 5 to 10 years to develop each additional clinical application of NIBS. Ultimately, she predicts that reigning in Alzheimer’s disease and mild cognitive impairment will require a multi-pronged approach that includes lifestyle and medications, too.
Sandrini believes that scientific efforts should focus on preventing or delaying Alzheimer’s. “We need to start intervention earlier—as soon as people start to complain about forgetting things,” he says. “Changes in the brain start 10 years before [there is a problem]. Once Alzheimer’s develops, it is too late.”
Will religious people reject organ transplants from pigs?
Scientists are getting better at transplanting pig organs into humans. But religious followers of prohibitions on consuming pork may reject these organs, even if their bodies could accept them.
The first successful recipient of a human heart transplant lived 18 days. The first artificial heart recipient lived just over 100.
Their brief post-transplant lives paved the way toward vastly greater successes. Former Vice President Dick Cheney relied on an artificial heart for nearly two years before receiving a human heart transplant. It still beats in his chest more than a decade later.
Organ transplantation recently reached its next phase with David Bennett. He survived for two months after becoming the first recipient of a pig’s heart genetically modified to function in a human body in February. Known as a xenotransplant, the procedure could pave the way for greatly expanding the use of transplanted vital organs to extend human lives.
Clinical trials would have to be held in the U.S. before xenotransplants become widespread; Bennett’s surgery was authorized under a special Food and Drug Administration program that addresses patients with life-threatening medical conditions.
German researchers plan to perform eight pig-to-human heart transplants as part of a clinical trial beginning in 2024. According to an email sent to Leaps.org by three scholars working on the German project, these procedures will focus on one of the reasons David Bennett did not survive longer: A porcine infection from his new heart.
The transplant team will conduct more sensitive testing of the donor organs, “which in all likelihood will be able to detect even low levels of virus in the xenograft,” note the scientists, Katharina Ebner, Jochen Ostheimer and Jochen Sautermeister. They are confident that the risk of infection with a porcine virus in the future will be significantly lower.
Moreover, hearts are not the only genetically modified organs that are being xenotransplanted. A team of surgeons at the University of Alabama at Birmingham successfully transplanted genetically modified pig kidneys into a brain-dead human recipient in September. The kidneys functioned normally for more than three days before the experiment ended. The UAB team is now moving forward with clinical trials focusing on transplanting pig kidneys into human patients.
Some experts believe the momentum for xenotransplantation is building, particularly given the recent successes. “I think there is a strong likelihood this will go mainstream,” says Brendan Parent of NYU Langone Health.
Douglas Anderson, a surgeon who is part of that kidney xenotransplant team, observes that, “organ shortages have been the major issue facing transplantation since its inception” and that xenotransplantation is a potential solution to that quandary. “It can’t be understated the number of people waiting for a kidney on dialysis, which has a significant mortality rate,” he says. According to the advocacy group Donate Life America, more than 100,000 people in the U.S. alone are waiting for a donated organ, and 85 percent of them need a kidney.
Other experts believe the momentum for xenotransplantation is building, particularly given the recent successes. “I think there is a strong likelihood this will go mainstream,” says Brendan Parent, director of transplant ethics and policy at NYU Langone Health, a New York City-based hospital system. Like the UAB team, surgeons at NYU Langone have had success coaxing modified pig kidneys to work in deceased humans.
“There is a genuinely good chance that within a generation, (xenotransplantation) might become very common in reasonably wealthy countries,” says Michael Reiss, professor of science education at University College in London. In addition to his academic position, Reiss sits on the Nuffield Council on Bioethics, a nonprofit that is one of Britain’s most prominent watchdogs regarding medical and scientific issues. Reiss is also an Anglican priest and has studied xenotransplantation from both a scientific and religious point of view.
Moreover, genetic modifications could one day lead to organs being specifically optimized for their recipients. That could ensure issues like donor rejection and the calculated risk of artificially suppressing recipient immune systems become concerns of the past.
Major bioethical, religious concerns
Despite the promise of xenotransplantation, numerous bioethical issues swirl around the procedure. They could be magnified if xenotransplantation evolves from one-off experiments to a routine medical procedure.
One of the biggest is the millennia-long prohibitions Islam and Judaism have had regarding the consumption of pork. Will followers of these religions assume such rules extend to those taboo materials being inserted into a human body?
“Initially, one’s instinctual reaction is that, oh, crumbs! – how are Jews and Muslims going to react to that?” Reiss says. But in a world where science and secularism are accepted on an everyday basis, he notes it is not a significant issue. Reiss points out that valves from pig hearts have been used in human patients for decades without any issues. He adds that both Islam and Judaism waive religious dietary restrictions if a human life is at risk.
“While nobody's saying an individual patient is to be forced to have these, the very high proportion of people who identify as Jews or Muslims when given this option are content with it,” he says.
Concurring with Reiss is Michael Gusamano, professor of health policy at Lehigh University and director of its Center for Ethics. He is currently performing research on the ethics of xenotransplantation for the National Institutes of Health.
“Leaders from all major religions have commented on this and have indicated that this is not inconsistent with religious doctrine,” Gusamano says in written remarks to Leaps.org. “Having said that, it is plausible to believe that some people will assume that this is inconsistent with the teaching of their religion and may object to…receiving a xenotransplant as part of routine medical care.”
A history of clashes
Despite those assurances, science has long clashed with theology. Although Galileo proved the planets revolved around the sun, the Catholic Church found him guilty of heresy and rewarded his discovery with house arrest for the last decade of his life. A revolt occurred in mid-19th century India after native-born soldiers believed the ammunition supplied by their British occupiers had been lubricated with pork and beef tallow. Given they had to use their mouths to tear open ammunition pouches, this violated both the tenets of Islam and Hinduism. And one of the conspiracy theories hatched as a result of COVID-19 was that the vaccines developed to fight the disease were the “mark of the beast” – a sign of impending Armageddon under evangelical Christian theology.
The German xenotransplant research team has encountered such potential concerns when the procedure is regarded through a religious lens. “The pastors in our research suspected that many recipients might feel disgust and revulsion,” they write. “Even beyond these special religious reservations, cultural scripts about pigs as inferior living beings are also generally widespread and effective in the western world, so that here too possible disgust reactions cannot be ruled out.”
The German researchers add that “Jewish and Muslim hospital pastoral workers believe possible considerable problems in this respect, which must be dealt with psychosocially, religiously, and pastorally prior to a possible transplantation in order to strengthen the acceptance of the received organ by the patients and their relatives.”
Parent, the director at NYU Langone, shares a concern that xenotransplantation could move “too fast,” although much of his worry is focused on zoonotic disease transmission – pig viruses jumping into humans as a result of such procedures.
Another ethical issue
Moreover, the way pigs and other animals are raised for transplants could pose future ethical dilemmas.
Reiss notes that pigs raised for medical procedures have to be grown and kept in what are known as a designated pathogen-free facility, or DPF. Such facilities are kept painstakingly antiseptic so as to minimize the risk of zoonotic transmissions. But given pigs are fond of outdoor activities such as wallowing in mud and sleeping on hay, they lead “stunningly boring lives” that they probably do not enjoy, Reiss observes.
Ethical concerns with using pigs may push transplantation medicine into its next logical phase: Growing functional organs for transplant in a laboratory setting.
“There’s no doubt that these research pigs have gotten much better veterinary care, et cetera, (compared to farmed pigs). But it’s not a great life,” Reiss says. “And although it hasn’t so far dominated the discussion, I think as the years go by, rather as we’ve seen with the use of apes and now monkeys in medical research, more and more theologians will get uncomfortable about us just assuming we can do this with…pigs.”
The German research team raises the same concerns, but has taken a fairly sanguine view on the topic. “The impairments of the species-typical behavior will certainly provoke criticism and perhaps also public protest. But the number of animals affected is very small in relation to slaughter cattle,” the German researchers note. “Moreover, the conditions there and also in several animal experiments are far worse.”
Observers say that may push transplantation medicine into its next logical phase: Growing functional organs for transplant in a laboratory setting. Anderson, the UAB transplant surgeon, believes such an accomplishment remains decades away.
But other experts believe there is a moral imperative that xenotransplantation remain a temporary solution. “I think we have a duty to go in that direction,” Parent says. “We have to go that way, with the xenotransplantation process (as) a steppingstone and research path that will be useful for bioengineered organs.”