What will the $100 genome mean?

A company has slashed the cost of assessing a person's genome to just $100. With lower costs - and as other genetic tools mature and evolve - a wave of new therapies could be coming in the near future.
In May 2022, Californian biotech Ultima Genomics announced that its UG 100 platform was capable of sequencing an entire human genome for just $100, a landmark moment in the history of the field. The announcement was particularly remarkable because few had previously heard of the company, a relative unknown in an industry long dominated by global giant Illumina which controls about 80 percent of the world’s sequencing market.
Ultima’s secret was to completely revamp many technical aspects of the way Illumina have traditionally deciphered DNA. The process usually involves first splitting the double helix DNA structure into single strands, then breaking these strands into short fragments which are laid out on a glass surface called a flow cell. When this flow cell is loaded into the sequencing machine, color-coded tags are attached to each individual base letter. A laser scans the bases individually while a camera simultaneously records the color associated with them, a process which is repeated until every single fragment has been sequenced.
Instead, Ultima has found a series of shortcuts to slash the cost and boost efficiency. “Ultima Genomics has developed a fundamentally new sequencing architecture designed to scale beyond conventional approaches,” says Josh Lauer, Ultima’s chief commercial officer.
This ‘new architecture’ is a series of subtle but highly impactful tweaks to the sequencing process ranging from replacing the costly flow cell with a silicon wafer which is both cheaper and allows more DNA to be read at once, to utilizing machine learning to convert optical data into usable information.
To put $100 genome in perspective, back in 2012 the cost of sequencing a single genome was around $10,000, a price tag which dropped to $1,000 a few years later. Before Ultima’s announcement, the cost of sequencing an individual genome was around $600.
Several studies have found that nearly 12 percent of healthy people who have their genome sequenced, then discover they have a variant pointing to a heightened risk of developing a disease that can be monitored, treated or prevented.
While Ultima’s new machine is not widely available yet, Illumina’s response has been rapid. In September 2022, the company unveiled the NovaSeq X series, which it describes as its fastest most cost-efficient sequencing platform yet, capable of sequencing genomes at $200, with further price cuts likely to follow.
But what will the rapidly tumbling cost of sequencing actually mean for medicine? “Well to start with, obviously it’s going to mean more people getting their genome sequenced,” says Michael Snyder, professor of genetics at Stanford University. “It'll be a lot more accessible to people.”
At the moment sequencing is mainly limited to certain cancer patients where it is used to inform treatment options, and individuals with undiagnosed illnesses. In the past, initiatives such as SeqFirst have attempted further widen access to genome sequencing based on growing amounts of research illustrating the potential benefits of the technology in healthcare. Several studies have found that nearly 12 percent of healthy people who have their genome sequenced, then discover they have a variant pointing to a heightened risk of developing a disease that can be monitored, treated or prevented.
“While whole genome sequencing is not yet widely used in the U.S., it has started to come into pediatric critical care settings such as newborn intensive care units,” says Professor Michael Bamshad, who heads the genetic medicine division in the University of Washington’s pediatrics department. “It is also being used more often in outpatient clinical genetics services, particularly when conventional testing fails to identify explanatory variants.”
But the cost of sequencing itself is only one part of the price tag. The subsequent clinical interpretation and genetic counselling services often come to several thousand dollars, a cost which insurers are not always willing to pay.
As a result, while Bamshad and others hope that the arrival of the $100 genome will create new opportunities to use genetic testing in innovative ways, the most immediate benefits are likely to come in the realm of research.
Bigger Data
There are numerous ways in which cheaper sequencing is likely to advance scientific research, for example the ability to collect data on much larger patient groups. This will be a major boon to scientists working on complex heterogeneous diseases such as schizophrenia or depression where there are many genes involved which all exert subtle effects, as well as substantial variance across the patient population. Bigger studies could help scientists identify subgroups of patients where the disease appears to be driven by similar gene variants, who can then be more precisely targeted with specific drugs.
If insurers can figure out the economics, Snyder even foresees a future where at a certain age, all of us can qualify for annual sequencing of our blood cells to search for early signs of cancer or the potential onset of other diseases like type 2 diabetes.
David Curtis, a genetics professor at University College London, says that scientists studying these illnesses have previously been forced to rely on genome-wide association studies which are limited because they only identify common gene variants. “We might see a significant increase in the number of large association studies using sequence data,” he says. “It would be far preferable to use this because it provides information about rare, potentially functional variants.”
Cheaper sequencing will also aid researchers working on diseases which have traditionally been underfunded. Bamshad cites cystic fibrosis, a condition which affects around 40,000 children and adults in the U.S., as one particularly pertinent example.
“Funds for gene discovery for rare diseases are very limited,” he says. “We’re one of three sites that did whole genome sequencing on 5,500 people with cystic fibrosis, but our statistical power is limited. A $100 genome would make it much more feasible to sequence everyone in the U.S. with cystic fibrosis and make it more likely that we discover novel risk factors and pathways influencing clinical outcomes.”
For progressive diseases that are more common like cancer and type 2 diabetes, as well as neurodegenerative conditions like multiple sclerosis and ALS, geneticists will be able to go even further and afford to sequence individual tumor cells or neurons at different time points. This will enable them to analyze how individual DNA modifications like methylation, change as the disease develops.
In the case of cancer, this could help scientists understand how tumors evolve to evade treatments. Within in a clinical setting, the ability to sequence not just one, but many different cells across a patient’s tumor could point to the combination of treatments which offer the best chance of eradicating the entire cancer.
“What happens at the moment with a solid tumor is you treat with one drug, and maybe 80 percent of that tumor is susceptible to that drug,” says Neil Ward, vice president and general manager in the EMEA region for genomics company PacBio. “But the other 20 percent of the tumor has already got mutations that make it resistant, which is probably why a lot of modern therapies extend life for sadly only a matter of months rather than curing, because they treat a big percentage of the tumor, but not the whole thing. So going forwards, I think that we will see genomics play a huge role in cancer treatments, through using multiple modalities to treat someone's cancer.”
If insurers can figure out the economics, Snyder even foresees a future where at a certain age, all of us can qualify for annual sequencing of our blood cells to search for early signs of cancer or the potential onset of other diseases like type 2 diabetes.
“There are companies already working on looking for cancer signatures in methylated DNA,” he says. “If it was determined that you had early stage cancer, pre-symptomatically, that could then be validated with targeted MRI, followed by surgery or chemotherapy. It makes a big difference catching cancer early. If there were signs of type 2 diabetes, you could start taking steps to mitigate your glucose rise, and possibly prevent it or at least delay the onset.”
This would already revolutionize the way we seek to prevent a whole range of illnesses, but others feel that the $100 genome could also usher in even more powerful and controversial preventative medicine schemes.
Newborn screening
In the eyes of Kári Stefánsson, the Icelandic neurologist who been a visionary for so many advances in the field of human genetics over the last 25 years, the falling cost of sequencing means it will be feasible to sequence the genomes of every baby born.
“We have recently done an analysis of genomes in Iceland and the UK Biobank, and in 4 percent of people you find mutations that lead to serious disease, that can be prevented or dealt with,” says Stefansson, CEO of deCODE genetics, a subsidiary of the pharmaceutical company Amgen. “This could transform our healthcare systems.”
As well as identifying newborns with rare diseases, this kind of genomic information could be used to compute a person’s risk score for developing chronic illnesses later in life. If for example, they have a higher than average risk of colon or breast cancer, they could be pre-emptively scheduled for annual colonoscopies or mammograms as soon as they hit adulthood.
To a limited extent, this is already happening. In the UK, Genomics England has launched the Newborn Genomes Programme, which plans to undertake whole-genome sequencing of up to 200,000 newborn babies, with the aim of enabling the early identification of rare genetic diseases.
"I have not had my own genome sequenced and I would not have wanted my parents to have agreed to this," Curtis says. "I don’t see that sequencing children for the sake of some vague, ill-defined benefits could ever be justifiable.”
However, some scientists feel that it is tricky to justify sequencing the genomes of apparently healthy babies, given the data privacy issues involved. They point out that we still know too little about the links which can be drawn between genetic information at birth, and risk of chronic illness later in life.
“I think there are very difficult ethical issues involved in sequencing children if there are no clear and immediate clinical benefits,” says Curtis. “They cannot consent to this process. I have not had my own genome sequenced and I would not have wanted my parents to have agreed to this. I don’t see that sequencing children for the sake of some vague, ill-defined benefits could ever be justifiable.”
Curtis points out that there are many inherent risks about this data being available. It may fall into the hands of insurance companies, and it could even be used by governments for surveillance purposes.
“Genetic sequence data is very useful indeed for forensic purposes. Its full potential has yet to be realized but identifying rare variants could provide a quick and easy way to find relatives of a perpetrator,” he says. “If large numbers of people had been sequenced in a healthcare system then it could be difficult for a future government to resist the temptation to use this as a resource to investigate serious crimes.”
While sequencing becoming more widely available will present difficult ethical and moral challenges, it will offer many benefits for society as a whole. Cheaper sequencing will help boost the diversity of genomic datasets which have traditionally been skewed towards individuals of white, European descent, meaning that much of the actionable medical information which has come out of these studies is not relevant to people of other ethnicities.
Ward predicts that in the coming years, the growing amount of genetic information will ultimately change the outcomes for many with rare, previously incurable illnesses.
“If you're the parent of a child that has a susceptible or a suspected rare genetic disease, their genome will get sequenced, and while sadly that doesn’t always lead to treatments, it’s building up a knowledge base so companies can spring up and target that niche of a disease,” he says. “As a result there’s a whole tidal wave of new therapies that are going to come to market over the next five years, as the genetic tools we have, mature and evolve.”
This article was first published by Leaps.org in October 2022.
New tech helps people of all ages stay social
Sonja Bauman, 39, and Mariela Florez, 83, taking one of their regular walks in Plantation, Fla. The pair met through an online platform called Papa that provides "family on demand." (Photo by Sonja Bauman)
In March, Sonja Bauman, 39, used an online platform called Papa, which offers “family on demand,” to meet Mariela Florez, an 83-year-old retiree. Despite living with her adult children, Florez was bored and lonely when they left for work, and her recoveries from a stroke and broken hip were going slowly. That's when Bauman began visiting twice per week. They take walks, strengthening Florez’s hip, and play games like Connect Four for mental stimulation. “It’s very important for me so I don’t feel lonely all day long,” said Florez. Her memories, blurred by the stroke, are gradually returning.
Papa is one of a growing number of tech approaches that are bringing together people of all ages. In addition to platforms like Papa that connect people in real life, other startups use virtual reality and video, with some of them focusing especially on deepening social connections between the generations — relationships that support the health of older and younger people alike. “I enjoy seeing Mariela as much as she enjoys seeing me,” Bauman said.
Connecting in real life
Telehealth expert Andrew Parker founded Papa in 2017 to improve the health outcomes of older adults and families. Seniors can meet people — some their grandkids’ age — for healthy activities, while working parents find retirees to watch their children. These “Papa Pals” are provided as a benefit through Medicare, Medicaid and some employer health plans.
In 2020, Papa connected Bauman, the 39-year-old Floridian, with another woman in her mid-70s who lives alone and has very limited mobility. Bauman began driving her to doctor’s appointments and helping her with chores around the house. “When I’m not there, she doesn’t leave her apartment,” said Bauman. The two have gone to the gym together, and they walk slowly through the neighborhood, chatting so it feels less like exercise.
Parker was driven to start Papa by the problem of social isolation among seniors, exacerbated by the pandemic, but he believes users of all ages can benefit. “Many of our Pals feel more comfortable opening up with older members than their same-aged friends,” he said.
Other platforms aim for similar, in-person connections. Generation Tech unites teens with seniors for technology training. And Mon Ami, which provides case management software for aging and disability service providers, has an app that connects isolated older people with college-age volunteers.
Making new connections through video
Several new sites match you with strangers for real-time video chatting on various topics, such as finding common ground on political issues. Other video platforms focus on intergenerational connections.
S. Jay Olshansky, a gerontology professor at the University of Illinois-Chicago, recalls the first time he saw Hyunseung Lee, an 11-year-old from Seoul, through his computer screen. The kid was shy, but Olshansky, 67, encouraged him to ask questions. “Turns out, he was thirsting for this kind of interaction.”
They’d connected through Eldera, a platform that pairs mentors age 60 and up with mentees, using an algorithm, for video conversations. “The time and wisdom of older adults is the most important natural resource we can give future generations,” said Dana Griffin, Eldera's CEO. “Connecting through a screen is the opposite of social media.”
In weekly meetings, Olshansky noticed Lee’s unique interest in math. “There’s something special in you,” Olshansky told him. “How do we bring it to the surface?” He suggested Lee write a book on his favorite subject, and the preteen ran with it, cranking out 70 pages in two weeks. Lee has published his love letter to theorems on Amazon.
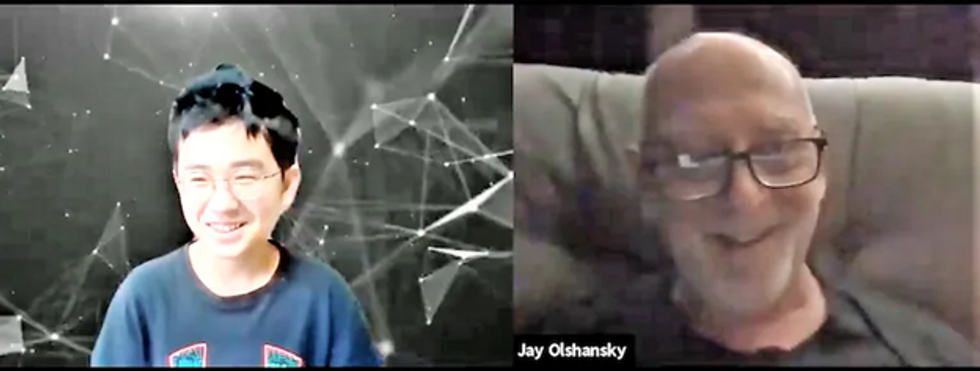
Hyunseung Lee, age 11, of Korea, and U.S. college professor Jay Olshanksy, 67, discuss math, strategy and Hyunsung's budding career as a book author during their video chats through a platform called Eldera. (Photo by Dana Palmer/Eldera)
Lee’s parents told Olshansky that their son has become more assertive — a recurring theme, Griffin said. “Confidence is the number one thing parents tell us about.” Since Eldera’s inception last year, the number of mentors has grown exponentially. Even so, Griffin said the waitlist for mentors typically numbers 200 kids.
Another site, Big and Mini, hosts video interactions between seniors and young adults; about 10,000 active users have joined since 2019, said co-founder Aditi Merchant.
Users often bring the benefits of their video interactions to their real-world relationships. Olshansky views Lee as an older version of his grandkids. “Eldera teaches me how to interact with them.” Lee, high on confidence, began instructing his classmates in math. Griffin noted that a group of Eldera mentors in Memphis, who met initially on Eldera, now take walks together in-person to trade ideas for helping each other’s Eldera kids solve problems in their schools and communities.
“We’ve evolved into a community for older adults who want to give back to the world,” said Griffin. Other new tools for connection take the form of virtual reality apps.
Connecting in virtual reality
During pandemic isolation, record numbers of people bought devices for virtual and augmented reality. Such gadgets can convince you that you’re hanging out with friends, even if they’re in another hemisphere. Lifelike simulations from miles away could be especially useful for meaningful interactions between people of different generations, since they’re often geographically segregated.
VR’s benefits require further study, but users report less social isolation and depression, according to MIT research. The immersive, 3-D experience is more compelling than FaceTime or Zoom. “It’s like the difference between a phone call and video call,” said Rick Robinson, Vice President of AARP’s Innovation Labs.
“When VR is designed right, the medium disappears,” said Jeremy Bailenson of Stanford.
Dana Pierce, a 56-year-old government employee in Indiana, got Meta's VR headset in May, 2021, thinking she’d enjoy it more than a new laptop. After many virtual group tours of exotic destinations, she has no regrets. Her adventures occur on Alcove, an app made by Robinson’s Innovation Labs. He co-created it with VR-company Rendever and sought input from people over age 50 to tailor it to their interests. “I’m an introvert,” said Pierce. “I’ve been more socially active since getting my headset than I am in real life.”
Tagging along with her to places like Paris are avatars representing real people around the world. She’s gotten to know VR users in their 70s, 80s and 90s, as well as younger people and some her own age. One is a new friend she plays chess with in relaxing nature settings. Another is her oldest son. He lives 90 minutes away but, earlier this year, Pierce welcomed him and his girlfriend to her virtual house on Alcove. They chatted in the living room decorated with family photos uploaded by Pierce. Then they took out a boat to go VR fishing — because why not — until 2 a.m.
“When VR is designed right, the medium disappears,” said Jeremy Bailenson, a communications professor who directs Stanford’s Virtual Human Interaction Lab. He’s teaching a class of 175 students entirely in VR. After months of covid isolation, the first time the class met, “there was a big catharsis. It really feels like you’re in a big crowd.” Like-minded people meet in VR for events such as comedy shows and creative writing meetups, while the Swedish pop group ABBA has performed this year as digital versions of themselves (“ABBA-tars”) during a virtual concert tour.
Karen Fingerman, a psychologist and director of the Texas Aging and Longevity Center at the University of Texas-Austin, supports the idea of VR for social connection, though she added that some people need it more than others. Hospitals and assisted-living facilities are using products such as Penumbra’s REAL I-Series and MyndVR to bring VR excursions to isolated patients and seniors. “If you’re in a bed or facility, this gives you something to talk about,” said Gita Barry, Penumbra’s executive vice president.
Pierce uses it on most days. She may see another adult son, who lives with her, less often as a result. But VR helps her manage real-world stressors, more than escaping them. After a long workday, she visits her back porch on Alcove, which overlooks a pond. “It’s my little retreat,” she said. “VR improves my mood. It’s added a lot to my life.”
Some seniors are using more than one technology. Olshansky and Lee discuss strategy while playing Internet chess. And Olshansky recently began using VR. He sees his sister, who lives far away, in a virtual beach house. “It’d be a great way to interact with Hyunseung,” he said. “I should get him a headset.”
A version of this article first appeared in The Washington Post on December 3, 2021.
Friday Five: These boots were made for walking, even for people who can't
New boots could have you moving like Iron Man, based on research this month by Stanford scientists.
The Friday Five covers important stories in health and science research that you may have missed - usually over the previous week but, today, we're doing a lookback on breakthrough research over the month of October. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
This Friday Five episode covers the following studies published and announced over the past month:
- New boots could have you moving like Iron Man
- The problem with bedtime munching
- The perfect recipe for tiny brains
- The best sports for kids to avoid lifelong health risks
- Can virtual reality reduce pain?

