Growing Human Organs Inside Pigs Could Save Lives, But the U.S. Won't Fund the Research

Pigs in a barn.
The shortage of organs is a public health menace. Approximately 120,000 people in the U.S. need a lifesaving organ transplant. Of those, approximately 75,000 patients are on the active waiting list. Every day, nearly 20 individuals die from the shortage of organs in the United States.
Ethical concerns about human-animal chimera research might be dramatically overblown.
Scientists worldwide are developing new methods with potential to save countless patients in need of organs. Such approaches have tremendous potential, if only ethical and regulatory challenges could be overcome first.
One way that scientists are proposing to increase the number of transplantable organs is to produce organs from patient stem cells. Owed to their ability to grow limitlessly in the lab and form all tissue types, pluripotent stem cells from patients, in principle, could supply an infinite amount of cells that could potentially be transplanted back into patients. Unfortunately, all efforts to generate organs that can be transplanted into patients from stem cells to date have been unsuccessful.
A different encouraging approach is to generate patient organs inside livestock species, such as pigs. In the latest methods, interspecies chimeras – animals containing cells from both humans and animals – are generated by introducing human stem cells into early-stage animal embryos. Key genes essential for organ formation are disabled, allowing the introduced human stem cells to fill the empty space. In theory, this strategy will produce a human organ inside pigs or sheep.
Creating chimeras is not new in biology. Chimeras, or animals comprised of tissues from two different individuals, have already been deployed in research. Mouse chimeras are routinely used to create genetically engineered mice to study genes. The concept of generating human organs inside pigs or sheep comes from previous studies involving interspecies chimeras generated between mice and rats. Past experiments have demonstrated that it is possible to generate a rat pancreas inside a mouse.
Scientific and Ethical Obstacles
Unfortunately, chimera research has faced hurdles that have impeded progress. Of note, attempts to generate interspecies chimeras by several groups have failed. The results of these studies indicate that human cells appear unable to grow inside mouse embryos. The levels of human chimerism – the number of human cells inside the host animal embryo – appear too low to support any human organ generation.
Another obstacle is that chimera generation is ethically controversial. Some question the moral status of an animal that is comprised of human and animal cells. The most concerning question is whether human cells will contribute to the host animal's brain, potentially altering the cognition of the animal. These issues have prompted scientists to proceed very cautiously with chimera experiments. However, such concerns might be dramatically overblown. This is because the levels of human chimerism are too low to cause any significant change in animal brain function.
The ethical controversy has affected research policy in the United States. In the United States, the National Institutes of Health (NIH), the major funding body of biomedical research, blocked funding for chimera research while ethical questions were considered. Later, it was proposed that a new review process would be instated for chimera research. However, no change in policy has actually happened. The restrictive NIH policy is a major barrier to chimera research progress because laboratories around the United States cannot obtain funding for it. Lifting the restrictions on NIH funding for chimera research would dramatically accelerate chimera research.
Nonetheless, despite the past and current hurdles that chimera research has faced, new advances are changing the landscape of chimera research.
It is time to lift restrictions on chimera research so that its promise can be fully realized.
Progress on the Horizon
Scientists are developing improved strategies to increase the numbers of cells in animal embryos to the point where it might be possible to generate a human organ in an animal. For example, it has been suggested that the human stem cells researchers have been using cannot grow in animals. Scientists have made advances in generating new types of human stem cells that might have an improved ability to form chimeras.
Additionally, scientists have identified some barriers responsible for the failure to generate chimeras. For example, preventing cell death and enhancing the ability of stem cells to compete with host animal tissues also improves the numbers of human cells to the point where human organs can be generated inside an animal.
Finally, a relaxation of regulatory hurdles in other countries has created a more permissive environment for human-animal interspecies chimera research. In March, the Japanese government approved the first such experiments that could comprise a new way of generating organs from patients for transplantation.
Additionally, in spite of the somewhat negative attention that chimera generation has received, the International Society for Stem Cell Research (ISSCR) supports the new Japanese policies allowing chimera experiments. The ISSCR maintains that research involving the generation of chimeras should be permitted, as long as rigorous oversight and ethics review occur.
Chimera research has the potential to transform medicine. Of all the impediments, the NIH restrictions on funding remain the single most significant barrier. It is time to lift restrictions on chimera research so that its promise can be fully realized. One day, it might be possible to grow patient-specific organs inside of livestock animals such as pigs and sheep, saving thousands of human lives. But to change our current policy, the public, scientists, and bioethicists must first agree that this critical cause is worth fighting for.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
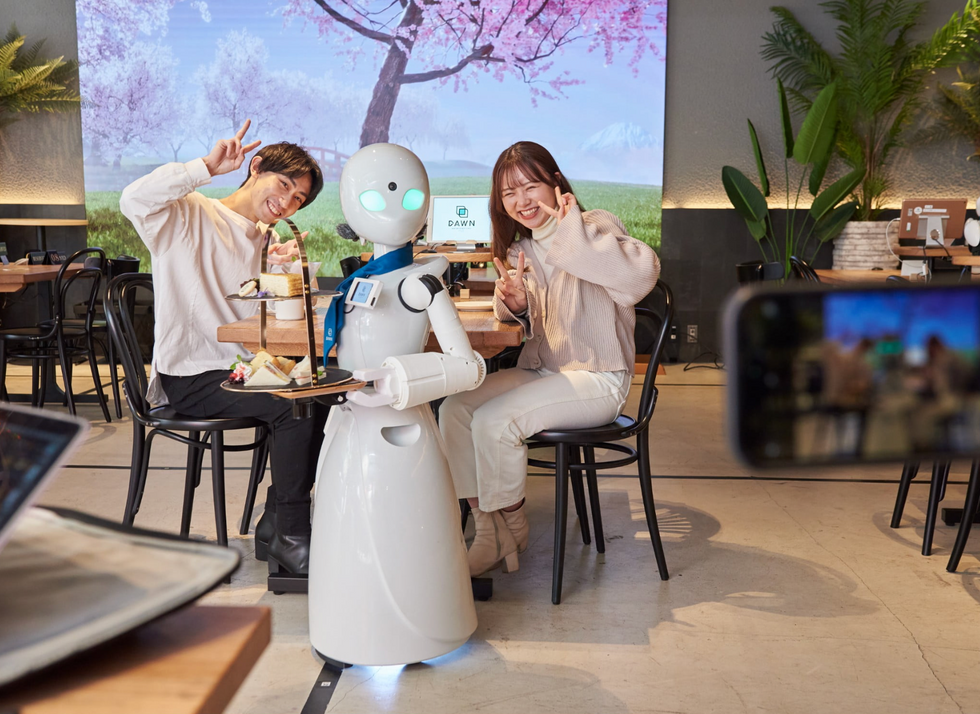
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

