Hacking Your Own Genes: A Recipe for Disaster

Employees of ODIN, a consumer genetic design and engineering company, working out of their Bay Area garage start-up lab in 2016.
Editor's Note: Our Big Moral Question this month is: "Where should we draw a line, if any, between the use of gene editing for the prevention and treatment of disease, and for cosmetic enhancement?" It is illegal in the U.S. to develop human trials for the latter, even though some people think it should be acceptable. The most outspoken supporter recently resorted to self-experimentation using CRISPR in his own makeshift lab. But critics argue that "biohackers" like him are recklessly courting harm. LeapsMag invited a leading intellectual from the Center for Genetics and Society to share her perspective.
"I want to democratize science," says biohacker extraordinaire Josiah Zayner.
This is certainly a worthy-sounding sentiment. And it is central to the ethos of biohacking, a term that's developed a bit of sprawl. Biohacking can mean non-profit community biology labs that promote "citizen science," or clever but not necessarily safe or innocuous garage-based experiments with computers and genetics, or efforts at biological self-optimization via techniques including cybernetic implants, drug supplements, and intermittent fasting.
They appear to have given little thought to whether curiosity should be bound in any way by care for social consequence.
Against that messy background, what should we make of Zayner? The thirty-something ex-NASA scientist, who describes himself as "a global leader in the BioHacker movement," put his interpretation of democracy on display last October during a CRISPR-yourself performance at a San Francisco biotech conference. In that episode, he dramatically jabbed himself with a long needle, injecting his left forearm with a home-made gene-editing concoction that he said would disrupt his myostatin genes and bulk up his muscles.
Zayner sees himself, and is seen by some fellow biohackers, as a rebel hero: an intrepid scientific adventurer willing to risk his own well-being in the tradition of self-experimentation, eager to push the boundaries of established science in the service of forging innovative modes of discovery, ready to stand up to those stodgy bureaucrats at the FDA in the name of biohacker freedom.
To others, including some in the biohacker community, he's a publicity-seeking stunt man, perhaps deluded by touches of toxic masculinity and techno-entrepreneurial ideology, peddling snake-oil with oozing ramifications.
Zayner is hardly coy about his goals being larger than Popeye-like muscles. "I want to live in a world where people are genetically modifying themselves," he told FastCompany. "I think this is, like, literally, a new era of human beings," he mused to CBS in November. "It's gonna create a whole new species of humans."
Nor does he deign to conceal his tactics. The webpage of the company he launched to sell DIY gene-editing kits (which is advised by celebrity geneticist George Church) says that Zayner is "constantly pushing the boundaries of Science outside traditional environments." He is more explicit when performing: "Yes I am a criminal. And my crime is that of curiosity," he said last August to a biohacker audience in Oakland, which according to Gizmodo erupted in applause.
Regrettably, Zayner, along with some other biohackers and their defenders in the mainstream scientific world, appear to have given little thought to whether curiosity should be bound in any way by care for social consequence.
In December, the FDA issued a brief statement warning against using DIY kits for self-administered gene editing.
Though what's most directly at risk in Zayner's self-enhancement hack is his own safety, his bad-boy celebrity status is likely to encourage emulation. A few weeks after his San Francisco performance, 27-year-old Tristan Roberts took to Facebook Live to give himself a DIY gene modification injection to keep his HIV infection in check, because he doesn't like taking the regular medications that prevent AIDS. Whatever it was that he put into his body was provided by a company that Gizmodo describes as a "mysterious biotech firm with transhumanist leanings."
Zayner doesn't outright provide DIY gene hacks to others. But among his company's offerings are a free DIY Human CRISPR Guide and a $20 CRISPR-Cas9 plasmid that targets the human myostatin gene – the one that Zayner said he was targeting to make his muscles grow. Presumably to fend off legal problems, the product page says: "This product is not injectable or meant for direct human use" – a label as toothless as the fine print on cigarette packages that breaks the news that smoking causes cancer.
Some scientists warn that Zayner's style of biohacking carries considerable dangers. Microbiologist Brian Hanley, himself a self-experimenter who now opposes "biohacking humans," focuses on the technical difficulty of purifying what's being injected. "Screwing up can kill you from endotoxin," he says. "If you get in trouble, call me. I will do my best to instruct the physician how to save your life….But I make no guarantees you will survive."
Hanley also commented on the likely effectiveness of Zayner's effort: "Either Josiah Zayner is ignorant or he is deliberately misleading people. What he suggests cannot work as advertised."
Ensuring the safety and effectiveness of medical drugs and devices is the mandate of the US Food and Drug Administration. In December, the agency issued a brief statement warning against using DIY kits for self-administered gene editing, and saying flat out that selling them is against the law.
The stem cell field provides an unfortunate model of what can go wrong.
Zayner is dismissive of the safety risks. He asks in a Buzzfeed article whether DIY CRISPR should be considered more harmful than smoking or chemotherapy, "legal and socially acceptable activities that damage your genes." This is a strange line of argument, given the decades-long battles with the tobacco industry to raise awareness about smoking's significant harms, and since the side effects of chemotherapy are typically not undertaken by choice.
But the implications of what Zayner, Roberts, and some of their fellow biohackers are promoting ripple well beyond direct harms to individuals. Their rhetoric and vision affect the larger project of biomedicine, and the fraught relationships among drug researchers, pharmaceutical companies, clinical trial subjects, patients, and the public. Writing in Scientific American, Eleanor Pauwels of the Wilson Center, who is sympathetic to biohacking, lists the down sides: "blurred boundaries between treatments and self-experimentation, peer pressure to participate in trials, exploitation of vulnerable individuals, lack of oversight concerning quality control and risk of harm, and more."
These prospects are germane to the current state of human gene editing. After decades of dashed hopes, including deaths of research subjects, "gene therapy" may now be close to deserving the promise in its name. But with safety and efficacy still being evaluated, it's especially crucial to be honest about limitations as well as possibilities.
The stem cell field provides an unfortunate model of what can go wrong. Fifteen years ago, scientists, patient advocates, and even politicians routinely indulged in wildly over-optimistic enthusiasm about the imminence of stem cell therapies. That binge of irresponsible promotion helped create the current situation of widespread stem cell fraud: hundreds of clinics in the US alone selling unproven treatments to unsuspecting and sometimes desperate patients. Many have had their wallets lightened; some have gone blind or developed strange tumors that doctors have never before seen. The FDA is scrambling to address this still-worsening situation.
Zayner-style biohacking and promotion may also impact the ongoing controversy about whether new gene editing tools should be used in human reproduction to pre-determine the traits of future children and generations. Much of the widespread opposition to "human germline modification" is grounded in concern that it would lead to a society in which real or purported genetic advantages, marketed by fertility clinics to affluent parents, would exacerbate our already shameful levels of inequality and discrimination.
With powerful new technologies increasingly shaping the world, there's a lot riding on our capacity to democratize science. But as a society we don't yet have much practice at it.
Yet Zayner is all for it. In an interview in The Guardian, he comments, "DNA defines what a species is, and I imagine it wouldn't be too long into the future when the human species almost becomes a new species because of these modifications." He notes in a blog post, "We want to grow as a species and maybe change as a species. Whether that is curing disease or immortality or mutant powers is up to you."
This brings us back to Zayner's claim that he is working to democratize science.
The conviction that gene editing involves social and political challenges, not just technical matters, has been voiced at all points on the spectrum of perspective and uncertainty. But Zayner says there's been enough talk. "I want people to stop arguing about whether it's okay to use CRISPR or not use CRISPR….It's too late: I already made the choice for you. Argument over. Let's get on with it now. Let's use this to help people. Or to give people purple skin." (Emphasis added, in case there's any doubt about Zayner's commitment to democracy.)
With powerful new technologies increasingly shaping the world, there's a lot riding on our capacity to democratize science. But as a society we don't yet have much practice at it. In fact, we're not very sure what it would look like. It would clearly mean, as Arizona State University political scientist David Guston puts it, "considering the societal outcomes of research at least as attentively as the scientific and technological outputs." It would need broad participation and demand hard work.
The involvement of serious citizen scientists in such efforts, biohackers included, could be a very good thing. But Zayner's contributions to date have not been helpful.
[Ed. Note: Check out Zayner's perspective: "Genetic Engineering for All: The Last Great Frontier of Human Freedom." Then follow LeapsMag on social media to share your opinion.]
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
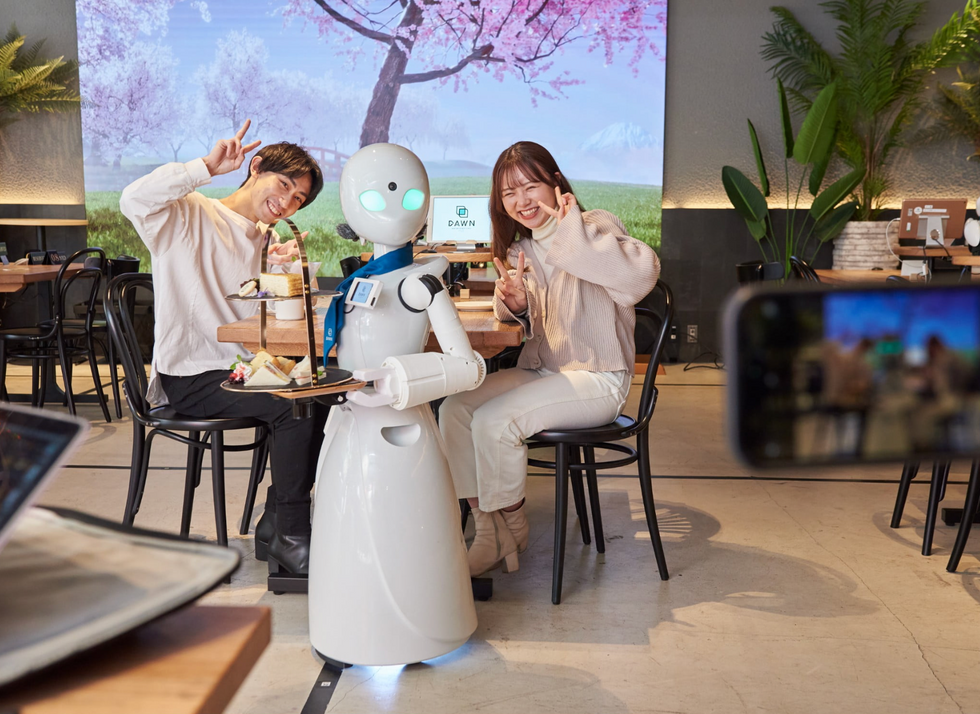
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
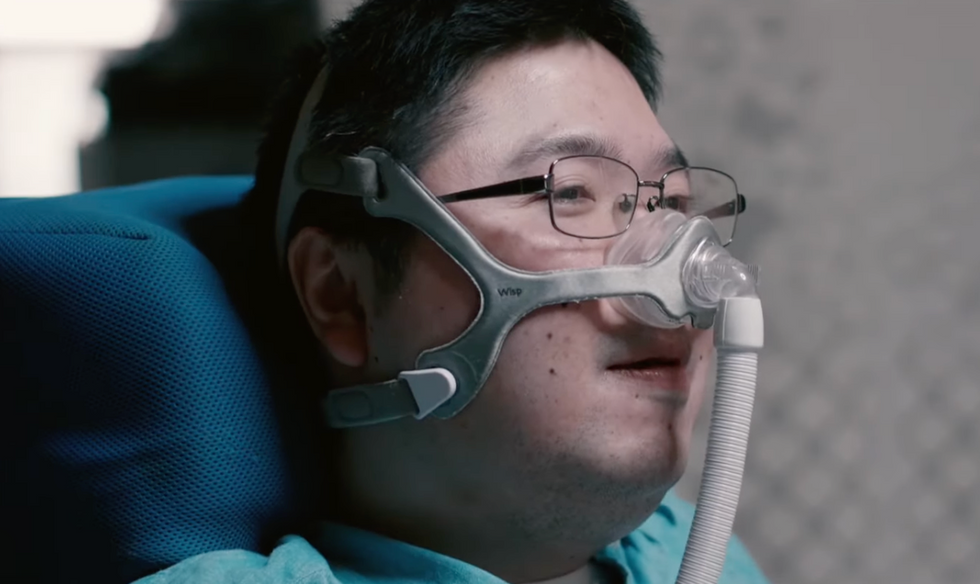
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

