Harvard Researchers Are Using a Breakthrough Tool to Find the Antibodies That Best Knock Out the Coronavirus
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
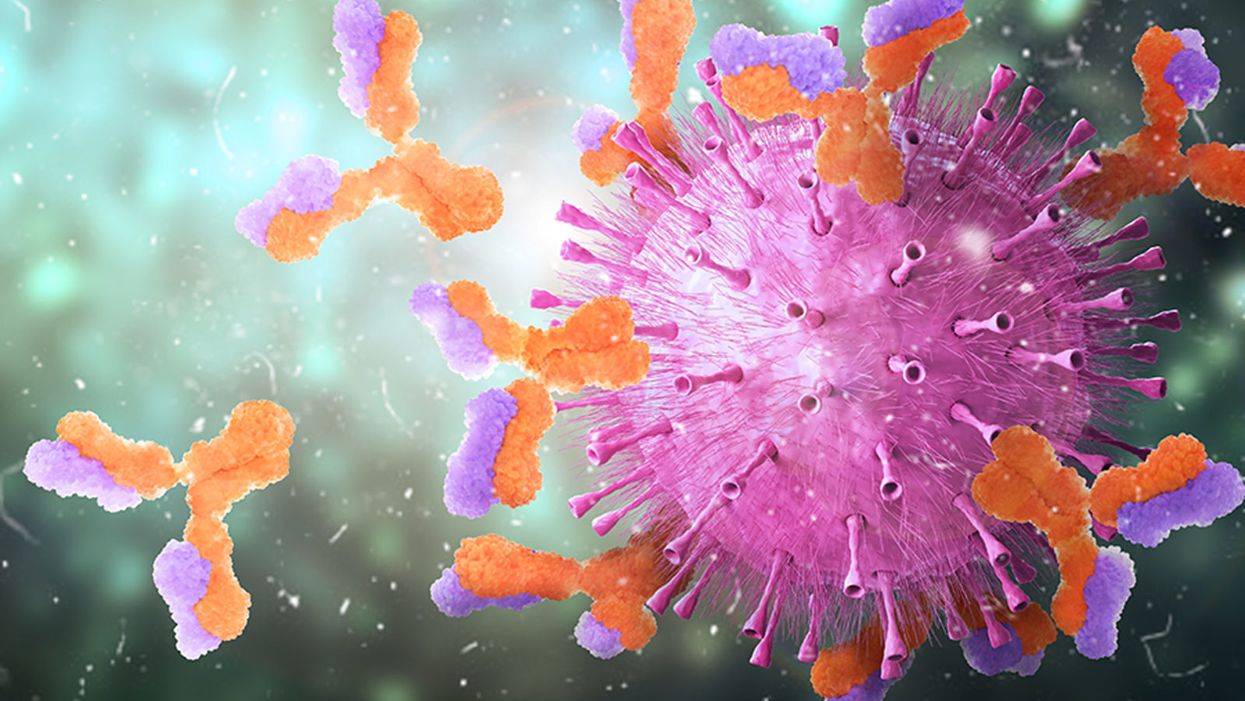
Knowing which antibodies bind best to the coronavirus can help guide new medicines and vaccine development.
To find a cure for a deadly infectious disease in the 1995 medical thriller Outbreak, scientists extract the virus's antibodies from its original host—an African monkey.
"When a person is infected, the immune system makes antibodies kind of blindly."
The antibodies prevent the monkeys from getting sick, so doctors use these antibodies to make the therapeutic serum for humans. With SARS-CoV-2, the original hosts might be bats or pangolins, but scientists don't have access to either, so they are turning to the humans who beat the virus.
Patients who recovered from COVID-19 are valuable reservoirs of viral antibodies and may help scientists develop efficient therapeutics, says Stephen J. Elledge, professor of genetics and medicine at Harvard Medical School in Boston. Studying the structure of the antibodies floating in their blood can help understand what their immune systems did right to kill the pathogen.
When viruses invade the body, the immune system builds antibodies against them. The antibodies work like Velcro strips—they use special spots on their surface called paratopes to cling to the specific spots on the viral shell called epitopes. Once the antibodies circulating in the blood find their "match," they cling on to the virus and deactivate it.
But that process is far from simple. The epitopes and paratopes are built of various peptides that have complex shapes, are folded in specific ways, and may carry an electrical charge that repels certain molecules. Only when all of these parameters match, an antibody can get close enough to a viral particle—and shut it out.
So the immune system forges many different antibodies with varied parameters in hopes that some will work. "When a person is infected, the immune system makes antibodies kind of blindly," Elledge says. "It's doing a shotgun approach. It's not sure which ones will work, but it knows once it's made a good one that works."
Elledge and his team want to take the guessing out of the process. They are using their home-built tool VirScan to comb through the blood samples of the recovered COVID-19 patients to see what parameters the efficient antibodies should have. First developed in 2015, the VirScan has a library of epitopes found on the shells of viruses known to afflict humans, akin to a database of criminals' mug shots maintained by the police.
Originally, VirScan was meant to reveal which pathogens a person overcame throughout a lifetime, and could identify over 1,000 different strains of viruses and bacteria. When the team ran blood samples against the VirScan's library, the tool would pick out all the "usual suspects." And unlike traditional blood tests called ELISA, which can only detect one pathogen at a time, VirScan can detect all of them at once. Now, the team has updated VirScan with the SARS-CoV-2 "mug shot" and is beginning to test which antibodies from the recovered patients' blood will bind to them.
Knowing which antibodies bind best can also help fine-tune vaccines.
Obtaining blood samples was a challenge that caused some delays. "So far most of the recovered patients have been in China and those samples are hard to get," Elledge says. It also takes a person five to 10 days to develop antibodies, so the blood must be drawn at the right time during the illness. If a person is asymptomatic, it's hard to pinpoint the right moment. "We just got a couple of blood samples so we are testing now," he said. The team hopes to get some results very soon.
Elucidating the structure of efficient antibodies can help create therapeutics for COVID-19. "VirScan is a powerful technology to study antibody responses," says Harvard Medical School professor Dan Barouch, who also directs the Center for Virology and Vaccine Research. "A detailed understanding of the antibody responses to COVID-19 will help guide the design of next-generation vaccines and therapeutics."
For example, scientists can synthesize antibodies to specs and give them to patients as medicine. Once vaccines are designed, medics can use VirScan to see if those vaccinated again COVID-19 generate the necessary antibodies.
Knowing which antibodies bind best can also help fine-tune vaccines. Sometimes, viruses cause the immune system to generate antibodies that don't deactivate it. "We think the virus is trying to confuse the immune system; it is its business plan," Elledge says—so those unhelpful antibodies shouldn't be included in vaccines.
More importantly, VirScan can also tell which people have developed immunity to SARS-CoV-2 and can return to their workplaces and businesses, which is crucial to restoring the economy. Knowing one's immunity status is especially important for doctors working on the frontlines, Elledge notes. "The resistant ones can intubate the sick."
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
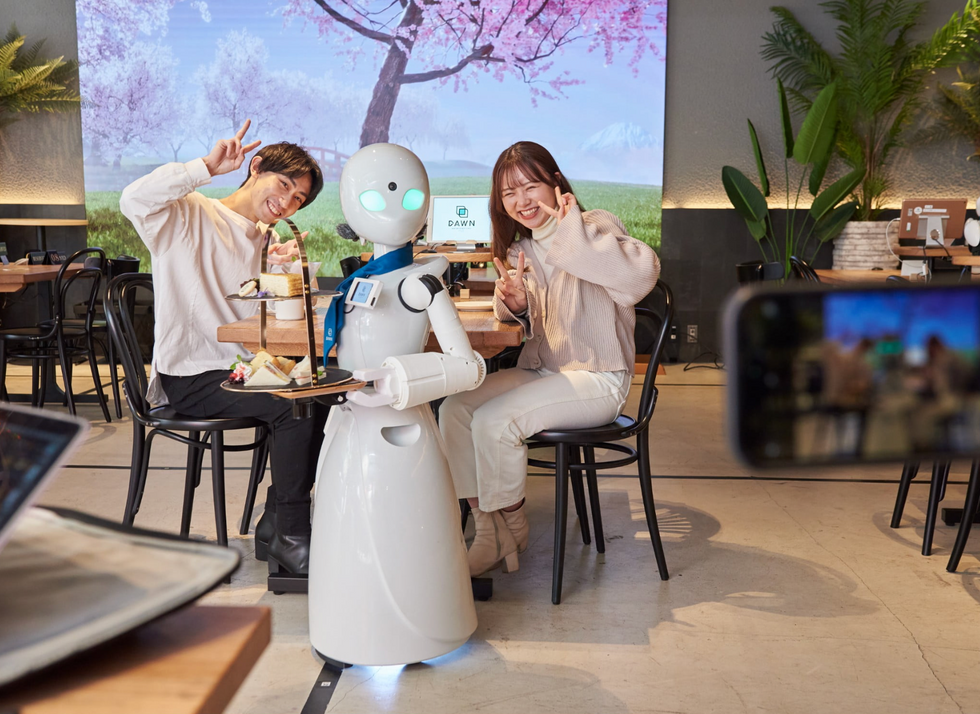
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
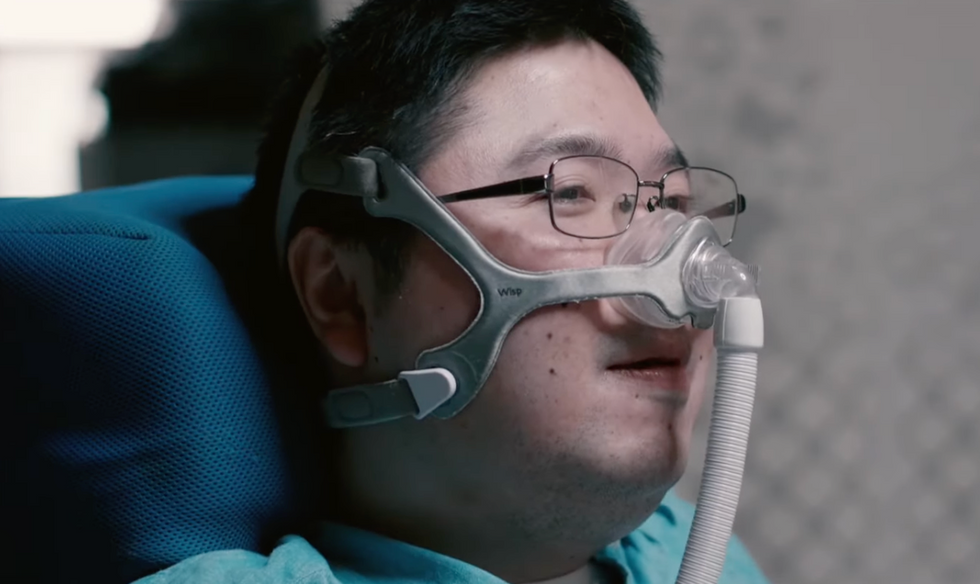
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

