Harvard Researchers Are Using a Breakthrough Tool to Find the Antibodies That Best Knock Out the Coronavirus
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
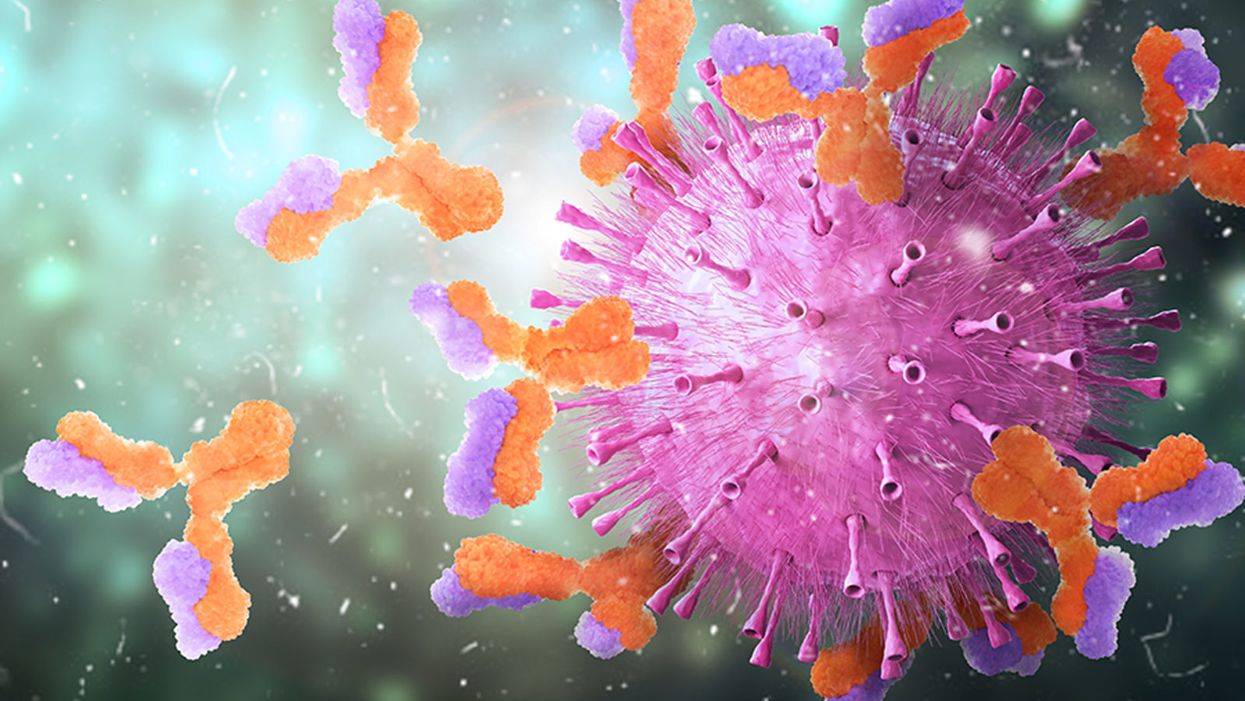
Knowing which antibodies bind best to the coronavirus can help guide new medicines and vaccine development.
To find a cure for a deadly infectious disease in the 1995 medical thriller Outbreak, scientists extract the virus's antibodies from its original host—an African monkey.
"When a person is infected, the immune system makes antibodies kind of blindly."
The antibodies prevent the monkeys from getting sick, so doctors use these antibodies to make the therapeutic serum for humans. With SARS-CoV-2, the original hosts might be bats or pangolins, but scientists don't have access to either, so they are turning to the humans who beat the virus.
Patients who recovered from COVID-19 are valuable reservoirs of viral antibodies and may help scientists develop efficient therapeutics, says Stephen J. Elledge, professor of genetics and medicine at Harvard Medical School in Boston. Studying the structure of the antibodies floating in their blood can help understand what their immune systems did right to kill the pathogen.
When viruses invade the body, the immune system builds antibodies against them. The antibodies work like Velcro strips—they use special spots on their surface called paratopes to cling to the specific spots on the viral shell called epitopes. Once the antibodies circulating in the blood find their "match," they cling on to the virus and deactivate it.
But that process is far from simple. The epitopes and paratopes are built of various peptides that have complex shapes, are folded in specific ways, and may carry an electrical charge that repels certain molecules. Only when all of these parameters match, an antibody can get close enough to a viral particle—and shut it out.
So the immune system forges many different antibodies with varied parameters in hopes that some will work. "When a person is infected, the immune system makes antibodies kind of blindly," Elledge says. "It's doing a shotgun approach. It's not sure which ones will work, but it knows once it's made a good one that works."
Elledge and his team want to take the guessing out of the process. They are using their home-built tool VirScan to comb through the blood samples of the recovered COVID-19 patients to see what parameters the efficient antibodies should have. First developed in 2015, the VirScan has a library of epitopes found on the shells of viruses known to afflict humans, akin to a database of criminals' mug shots maintained by the police.
Originally, VirScan was meant to reveal which pathogens a person overcame throughout a lifetime, and could identify over 1,000 different strains of viruses and bacteria. When the team ran blood samples against the VirScan's library, the tool would pick out all the "usual suspects." And unlike traditional blood tests called ELISA, which can only detect one pathogen at a time, VirScan can detect all of them at once. Now, the team has updated VirScan with the SARS-CoV-2 "mug shot" and is beginning to test which antibodies from the recovered patients' blood will bind to them.
Knowing which antibodies bind best can also help fine-tune vaccines.
Obtaining blood samples was a challenge that caused some delays. "So far most of the recovered patients have been in China and those samples are hard to get," Elledge says. It also takes a person five to 10 days to develop antibodies, so the blood must be drawn at the right time during the illness. If a person is asymptomatic, it's hard to pinpoint the right moment. "We just got a couple of blood samples so we are testing now," he said. The team hopes to get some results very soon.
Elucidating the structure of efficient antibodies can help create therapeutics for COVID-19. "VirScan is a powerful technology to study antibody responses," says Harvard Medical School professor Dan Barouch, who also directs the Center for Virology and Vaccine Research. "A detailed understanding of the antibody responses to COVID-19 will help guide the design of next-generation vaccines and therapeutics."
For example, scientists can synthesize antibodies to specs and give them to patients as medicine. Once vaccines are designed, medics can use VirScan to see if those vaccinated again COVID-19 generate the necessary antibodies.
Knowing which antibodies bind best can also help fine-tune vaccines. Sometimes, viruses cause the immune system to generate antibodies that don't deactivate it. "We think the virus is trying to confuse the immune system; it is its business plan," Elledge says—so those unhelpful antibodies shouldn't be included in vaccines.
More importantly, VirScan can also tell which people have developed immunity to SARS-CoV-2 and can return to their workplaces and businesses, which is crucial to restoring the economy. Knowing one's immunity status is especially important for doctors working on the frontlines, Elledge notes. "The resistant ones can intubate the sick."
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
Urinary tract infections account for more than 8 million trips to the doctor each year.
Few things are more painful than a urinary tract infection (UTI). Common in men and women, these infections account for more than 8 million trips to the doctor each year and can cause an array of uncomfortable symptoms, from a burning feeling during urination to fever, vomiting, and chills. For an unlucky few, UTIs can be chronic—meaning that, despite treatment, they just keep coming back.
But new research, presented at the European Association of Urology (EAU) Congress in Paris this week, brings some hope to people who suffer from UTIs.
Clinicians from the Royal Berkshire Hospital presented the results of a long-term, nine-year clinical trial where 89 men and women who suffered from recurrent UTIs were given an oral vaccine called MV140, designed to prevent the infections. Every day for three months, the participants were given two sprays of the vaccine (flavored to taste like pineapple) and then followed over the course of nine years. Clinicians analyzed medical records and asked the study participants about symptoms to check whether any experienced UTIs or had any adverse reactions from taking the vaccine.
The results showed that across nine years, 48 of the participants (about 54%) remained completely infection-free. On average, the study participants remained infection free for 54.7 months—four and a half years.
“While we need to be pragmatic, this vaccine is a potential breakthrough in preventing UTIs and could offer a safe and effective alternative to conventional treatments,” said Gernot Bonita, Professor of Urology at the Alta Bro Medical Centre for Urology in Switzerland, who is also the EAU Chairman of Guidelines on Urological Infections.
The news comes as a relief not only for people who suffer chronic UTIs, but also to doctors who have seen an uptick in antibiotic-resistant UTIs in the past several years. Because UTIs usually require antibiotics, patients run the risk of developing a resistance to the antibiotics, making infections more difficult to treat. A preventative vaccine could mean less infections, less antibiotics, and less drug resistance overall.
“Many of our participants told us that having the vaccine restored their quality of life,” said Dr. Bob Yang, Consultant Urologist at the Royal Berkshire NHS Foundation Trust, who helped lead the research. “While we’re yet to look at the effect of this vaccine in different patient groups, this follow-up data suggests it could be a game-changer for UTI prevention if it’s offered widely, reducing the need for antibiotic treatments.”
MILESTONE: Doctors have transplanted a pig organ into a human for the first time in history
A surgeon at Massachusetts General Hospital prepares a pig organ for transplant.
Surgeons at Massachusetts General Hospital made history last week when they successfully transplanted a pig kidney into a human patient for the first time ever.
The recipient was a 62-year-old man named Richard Slayman who had been living with end-stage kidney disease caused by diabetes. While Slayman had received a kidney transplant in 2018 from a human donor, his diabetes ultimately caused the kidney to fail less than five years after the transplant. Slayman had undergone dialysis ever since—a procedure that uses an artificial kidney to remove waste products from a person’s blood when the kidneys are unable to—but the dialysis frequently caused blood clots and other complications that landed him in the hospital multiple times.
As a last resort, Slayman’s kidney specialist suggested a transplant using a pig kidney provided by eGenesis, a pharmaceutical company based in Cambridge, Mass. The highly experimental surgery was made possible with the Food and Drug Administration’s “compassionate use” initiative, which allows patients with life-threatening medical conditions access to experimental treatments.
The new frontier of organ donation
Like Slayman, more than 100,000 people are currently on the national organ transplant waiting list, and roughly 17 people die every day waiting for an available organ. To make up for the shortage of human organs, scientists have been experimenting for the past several decades with using organs from animals such as pigs—a new field of medicine known as xenotransplantation. But putting an animal organ into a human body is much more complicated than it might appear, experts say.
“The human immune system reacts incredibly violently to a pig organ, much more so than a human organ,” said Dr. Joren Madsen, director of the Mass General Transplant Center. Even with immunosuppressant drugs that suppress the body’s ability to reject the transplant organ, Madsen said, a human body would reject an animal organ “within minutes.”
So scientists have had to use gene-editing technology to change the animal organs so that they would work inside a human body. The pig kidney in Slayman’s surgery, for instance, had been genetically altered using CRISPR-Cas9 technology to remove harmful pig genes and add human ones. The kidney was also edited to remove pig viruses that could potentially infect a human after transplant.
With CRISPR technology, scientists have been able to prove that interspecies organ transplants are not only possible, but may be able to successfully work long term, too. In the past several years, scientists were able to transplant a pig kidney into a monkey and have the monkey survive for more than two years. More recently, doctors have transplanted pig hearts into human beings—though each recipient of a pig heart only managed to live a couple of months after the transplant. In one of the patients, researchers noted evidence of a pig virus in the man’s heart that had not been identified before the surgery and could be a possible explanation for his heart failure.
So far, so good
Slayman and his medical team ultimately decided to pursue the surgery—and the risk paid off. When the pig organ started producing urine at the end of the four-hour surgery, the entire operating room erupted in applause.
Slayman is currently receiving an infusion of immunosuppressant drugs to prevent the kidney from being rejected, while his doctors monitor the kidney’s function with frequent ultrasounds. Slayman is reported to be “recovering well” at Massachusetts General Hospital and is expected to be discharged within the next several days.