He Wants to Eliminate Inherited Diseases in Embryos. Congress Just Said No (Again).
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Dr. Shoukhrat Mitalipov, May 13, 2013.
Biologist Shoukhrat Mitalipov is famous—and controversial--in the world of cutting-edge fertility treatments. A decade ago, he pioneered mitochondrial replacement therapy, paving the way for the world's first "three-parent" babies to be born free of a devastating inherited disease.
He sees his work toward embryo gene therapy as not only moral, but necessary.
In 2017, he shocked the world again when his group at Oregon Health and Science University became the first to repair a genetic mutation causing heart disease in dozens of human embryos. The embryos were later destroyed a part of the experiment; current policy in the U.S. prohibits such research from moving into clinical trials.
And that policy doesn't look like it's going to change anytime soon, despite recent political wavering. Last month, a House subcommittee dropped the ban that has blocked the Food and Drug Administration since 2015 from considering any clinical trials of genetically altered embryos intended to create a baby. The move raised the hopes of supporters who want to see such research move forward and angered critics who feel that the science is getting ahead of the ethics. But yesterday, a House committee decided to restore the ban on gene-edited babies after all.
As for Mitalipov, he told leapsmag that he sees his work toward embryo gene therapy as not only moral, but necessary. This interview has been edited and condensed for clarity.
What motivates you to pursue this line of research, even though it is highly controversial?
It's my expertise, I'm an embryologist. We study early development in humans -- sperm, egg, and the first five days of development -- and try to use our knowledge to treat human diseases, particularly in that early stage. This is how IVF started, as a treatment for infertility. It's a very successful cell therapy treatment, with millions of children born. [Now the idea is] to actually to use this IVF platform not as much to treat infertility, but also to treat heritable genetic diseases, because this is a very important stage when gametes from either dad or mom will transmit mutations. This is the bottleneck where we could actually interfere and repair that mutation.
Many people are hesitant to support embryo editing because of "designer babies," yet polls do show that Americans are more open to embryo editing for the purpose of disease prevention. Where should society draw a line?
Yeah, I agree with most Americans that we don't have to edit -- meaning you could make all kind of changes. Instead we do gene repair, which is a therapeutic application.
Gene repair is quite different than gene editing. It involves [focusing on] already known disease-causing mutations and how we can turn them back to normal.
Thousands of gene mutations cause human diseases, like Crohn's, for example, or mutations causing cancer, heart disease. These are well-described, well-studied cause-and-effect diseases and we need to do something about it because otherwise it's impossible to treat once the mutation is already passed to a child.
Early intervention is the best in any disease, but in genetics, "early" means you have to do it at the time of fertilization. That's when we are dealing with one copy of the mutation or maybe two, versus when you have a whole body with billions of cells in solid tissues that we cannot really access and target. So this is the most efficient way of preventing thousands and thousands of genetic diseases. I understand that we have to make sure that it's very safe, of course, and efficient as well. But at the same time, I think this is the future. We have to work toward developing these technologies.
"If we continue banning the research everywhere and not funding it, maybe 100 years will not be enough."
What's your opinion of Dr. He Jiankui and the Chinese CRISPR'ed babies?
This is a case where he was doing gene editing, not gene repair. He hasn't corrected anything, he induced a mutation to normal human genes, hoping that this would somehow confer resistance to HIV, which is still unclear.
I think such straightforward editing is unacceptable specifically for human embryos. He's approach has also never been tested in an animal model. That's why the reaction from the public and scientists was very negative, because this is the case where the doctor does this without any expertise in this area, without knowing probably much about what he is doing, and he acquired it without any oversights, which is troubling. And of course, it negatively affects the legitimate research that is going on in some labs.
What might the future of embryo gene therapy look like?
Hopefully in 10 years from now, thousands and thousands of families that know they carry germline mutations…could go through IVF and we would correct it, and they could have healthy children.
Right now, we have some tools. We cannot correct, but we can select. So what happens is the parents become pregnant and then at about three months along, we can biopsy the amniotic fluid and say, "Hey unfortunately you passed on this mutation." And that means this child, if it's born, will be affected, so we give parents a choice of terminating the pregnancy.
Or we could do it much earlier, so parents go to the IVF clinic where we retrieve about ten eggs, after stimulating a woman's ovaries. Each of them will be fertilized so we have ten embryos that develop. We have a five-day window where we can keep them in the lab. And we basically reap a few cells, we do a biopsy from each of these ten, and we say, "Hey embryo number 1 and number 4 are not mutant, but the others are."
Then we can take these two and the other eight usually will be thrown away. That's the technology that we have now. Some ethicists argue on religious grounds that we have this selection technology available, so why do we need germline gene therapy [i.e. repairing the disease-causing mutations in an embryo]?
I don't understand the moral argument there, because all the available technology is based on selective destruction of the embryo.
With [IVF gene therapy], we will take ten embryos and every embryo we'll make healthy because we can get rid of the mutations. How could embryo destruction be morally superior?
How long do you think it will take for this technology to be available to prospective parents?
It depends how many legitimate labs with expertise can get into this field and resolve all the scientific questions. If we continue banning the research everywhere and not funding it, maybe 100 years will not be enough.
So far, I think that my lab is the only one legitimately working on it. But we would like five, 10, maybe 100 labs in this country and Europe really working. Because we have scientific challenges that we need to resolve before we could say, "Hey now we know how to correct [a given mutation] and now this could be efficient, and there are no side effects or very little." And then we could say, "Okay, I think we've done everything we could in petri dishes and in animals, and now we are ready to transplant this embryo in a patient and see what happens."
"There's just no way you could sink your head into the sand and say, 'Oh, we just ban it and then hopefully everything will go away.'"
Does banning emerging technology actually work?
Banning it usually means it will leak out to a gray area where there's no regulation and many private IVF clinics will just use it while it is still premature. So I think we have to regulate the clinical testing. There's just no way you could sink your head into the sand and say, "Oh, we just ban it and then hopefully everything will go away." That's not going to happen.
If this technology does become feasible and legal in the future, do you think that more and more couples will choose IVF and gene therapy versus the natural method of rolling the dice?
As sequencing technology is becoming available, like 23andMe, more and more parents will realize what kind of mutations they carry. And if your spouse carries the same mutation on the same locus, now you have very high chance of transmitting it. Most of the time today, we find out these families carry it once they have one or two children with that condition.
Of course, parents can just do it naturally in the bedroom and have a chance of transmitting or not transmitting mutations, but hopefully eventually we can say, "Hey, because of your condition, you don't want to play this Russian Roulette. Let's just do IVF." And hopefully the government will cover that kind of treatment because right now IVF is not covered in most states. And we do this therapy and then they have a healthy child.
We have 10,000 different mutations in the human population. That means probably billions of people carry mutations. And unless they go through this gene therapy through IVF, they will keep transmitting them. And we're going to keep having millions and millions of children with diseases. We have to do something about it.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
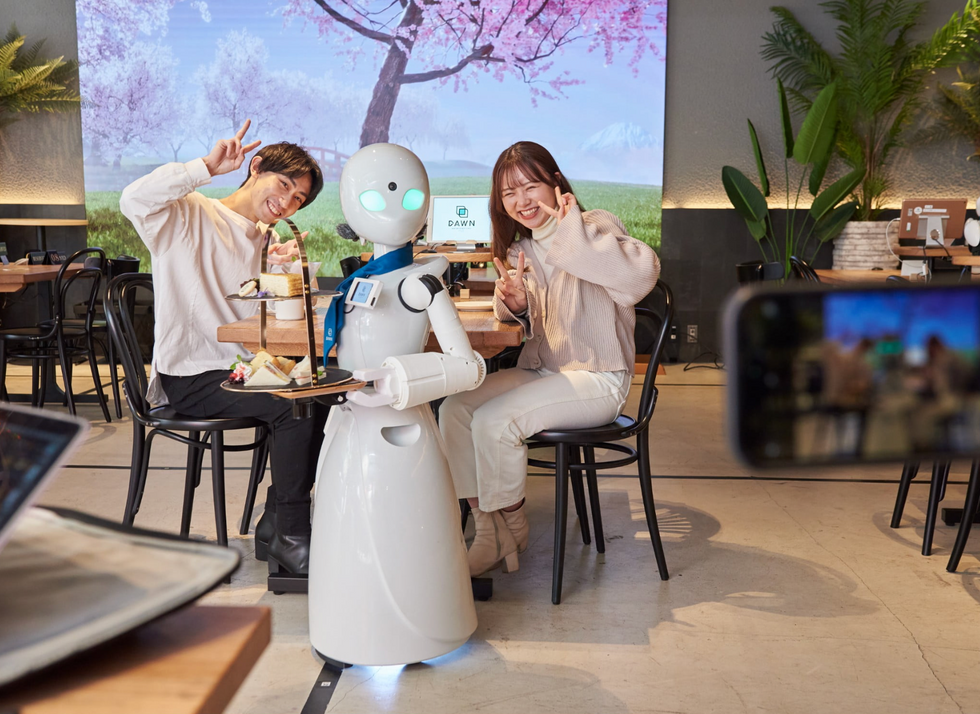
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

