How One Doctor Single-Handedly Saved Countless Babies from Birth Defects

President John F. Kennedy gave Dr. Frances Oldham Kelsey the nation's highest federal civilian service award in 1962, saying she had "prevented a major tragedy of birth deformities."
In July 1956, a new drug hit the European market for the first time. The drug was called thalidomide – a sedative that was considered so safe it was available without a prescription.
Sedatives were in high demand in post-war Europe – but barbiturates, the most widely-used sedative at the time, caused overdoses and death when consumers took more than the recommended amount. Thalidomide, on the other hand, didn't appear to cause any side effects at all: Chemie Grünenthal, thalidomide's manufacturer, dosed laboratory rodents with over 600 times the normal dosage during clinical testing and had observed no evidence of toxicity.
The drug therefore was considered universally safe, and Grünenthal supplied thousands of doctors with samples to give to their patients. Doctors were encouraged to recommend thalidomide to their pregnant patients specifically because it was so safe, in order to relieve the nausea and insomnia associated with the first trimester of pregnancy.
By 1960, Thalidomide was being sold in countries throughout the world, and the United States was expected to soon follow suit. Dr. Frances Oldham Kelsey, a pharmacologist and physician, was hired by the Food and Drug Administration (FDA) in September of that year to review and approve drugs for the administration. Immediately, Kelsey was tasked with approving thalidomide for commercial use in the United States under the name Kevadon. Kelsey's approval was supposed to be a formality, since the drug was so widely used in other countries.
But Kelsey did something that few people expected – she paused. Rather than approving the drug offhand as she was expected to do, Kelsey asked the manufacturer – William S. Merrell Co., who was manufacturing thalidomide under license from Chemie Grünenthal – to supply her with more safety data, noting that Merrell's application for approval relied mostly on anecdotal testimony. Kelsey – along with her husband who worked as a pharmacologist at the National Institutes of Health (NIH) — was highly suspicious of a drug that had no lethal dose and no side effects. "It was just too positive," Kelsey said later. "This couldn't be the perfect drug with no risk."
At the same time, rumors were starting to swirl across Europe that thalidomide was not as safe as everyone had initially thought: Physicians were starting to notice an "unusual increase" in the birth of severely deformed babies, and they were beginning to suspect thalidomide as the cause. The babies, whose mothers had all taken thalidomide during pregnancy, were born with conditions like deafness, blindness, congenital heart problems, and even phocomelia, a malformation of the arms and legs. Doctors and midwives were also starting to notice a sharp rise in miscarriages and stillbirths among their patients as well.
Kelsey's skepticism was rewarded in November 1961 when thalidomide was yanked abruptly off the market, following a growing outcry that it was responsible for hundreds of stillbirths and deformities.
Kelsey had heard none of these rumors, but she did know from her post-doctoral research that adults could metabolize drugs differently than fetuses – in other words, a drug that was perfectly safe for adults could be detrimental to a patient's unborn child. Noting that thalidomide could cross the placental barrier, she asked for safety data, such as clinical trials, that showed specifically the drug was non-toxic for fetuses. Merrell supplied Kelsey with anecdotal data – in other words, accounts from patients who attested to the fact that they took thalidomide with no adverse effects – but she rejected it, needing stronger data: clinical studies with pregnant women included.
The drug company was annoyed at what they considered Kelsey's needless bureaucracy. After all, Germans were consuming around 1 million doses of thalidomide every day in 1960, with lots of anecdotal evidence that it was safe, even among pregnant women. As the holidays approached – the most lucrative time of year for sedative sales – Merrell executives started hounding Kelsey to approve thalidomide, even phoning her superior and paying her visits at work. But Kelsey was unmovable. Kelsey's skepticism was solidified in December 1960, when she read a letter published in the British Medical Journal from a physician. In the letter, the author warned that his long-term thalidomide patients were starting to report pain in their arms and legs.
"The burden of proof that the drug is safe … lies with the applicant," Kelsey wrote in a letter to Merrell executive Joseph F. Murray in May of 1961. Despite increasing pressure, Kelsey held fast to her insistence that more safety data – particularly for fetuses – was needed.
Kelsey's skepticism was rewarded in November 1961 when Chemie Grünenthal yanked thalidomide off the market overseas, following a growing outcry that it was responsible for hundreds of stillbirths and deformities. In early 1962, Merrell conceded that the drug's safety was unproven in fetuses and formally withdrew its application at the FDA.
Thanks to Kelsey, the United States was spared the effects of thalidomide – although countries like Europe and Canada were not so lucky. Thalidomide remained in people's homes under different names long after it was pulled from the market, and so women unfortunately continued to take thalidomide during their pregnancies, unaware of its effects. All told, thalidomide is thought to have caused around 10,000 birth defects and anywhere from 5,000 to 7,000 miscarriages. Many so-called "thalidomide babies" are now adults living with disabilities.

Niko von Glasow, born in 1960, is a German film director and producer who was born disabled due to the side effects of thalidomide.
Wikimedia Commons
Just two years after joining the FDA, Kelsey was presented with the President's Award for Distinguished Federal Civilian Service and was appointed as the head of the Investigational Drug Branch at the FDA. Not only did Kelsey save the U.S. public from the horrific effects of thalidomide, but she forever changed the way drugs were developed and approved for use in the United States: Drugs now need to not only be proven safe and effective, but adverse drug reactions need to be reported to the FDA and informed consent must be obtained by all participants before they volunteer for clinical trials. Today, the United States is safer because of Frances Kelsey's bravery.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
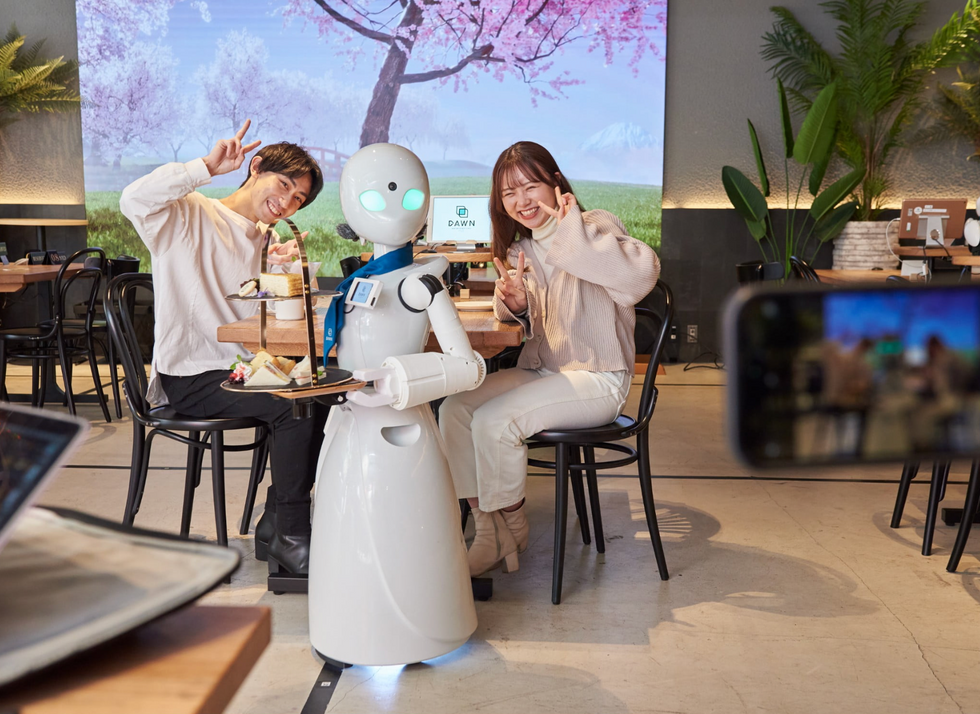
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

