Is It Possible to Predict Your Face, Voice, and Skin Color from Your DNA?

A slide from J. Craig Venter's recent study on facial prediction presented at the Summit Conference in Los Angeles on Nov. 3, 2017.
Renowned genetics pioneer Dr. J Craig Venter is no stranger to controversy.
Back in 2000, he famously raced the public Human Genome Project to decode all three billion letters of the human genome for the first time. A decade later, he ignited a new debate when his team created a bacterial cell with a synthesized genome.
Most recently, he's jumped back into the fray with a study in the September issue of the Proceedings of the National Academy of Sciences about the predictive potential of genomic data to identify individual traits such as voice, facial structure and skin color.
The new study raises significant questions about the privacy of genetic data.
His study applied whole-genome sequencing and statistical modeling to predict traits in 1,061 people of diverse ancestry. His approach aimed to reconstruct a person's physical characteristics based on DNA, and 74 percent of the time, his algorithm could correctly identify the individual in a random lineup of 10 people from his company's database.
While critics have been quick to cast doubt on the plausibility of his claims, the ability to discern people's observable traits, or phenotypes, from their genomes may grow more precise as technology improves, raising significant questions about the privacy and usage of genetic information in the long term.

J. Craig Venter showing slides from his recent study on facial prediction at the Summit Conference in Los Angeles on Nov. 3, 2017.
(Courtesy of Kira Peikoff)
Critics: Study Was Incomplete, Problematic
Before even redressing these potential legal and ethical considerations, some scientists simply said the study's main result was invalid. They pointed out that the methodology worked much better in distinguishing between people of different ethnicities than those of the same ethnicity. One of the most outspoken critics, Yaniv Erlich, a geneticist at Columbia University, said, "The method doesn't work. The results were like, 'If you have a lineup of ten people, you can predict eight."
Erlich, who reviewed Venter's paper for Science, where it was rejected, said that he came up with the same results—correctly predicting eight of ten people—by just looking at demographic factors such as age, gender and ethnicity. He added that Venter's recent rebuttal to his criticism was that 'Once we have thousands of phenotypes, it might work better.' But that, Erlich argued, would be "a major breach of privacy. Nobody has thousands of phenotypes for people."
Other critics suggested that the study's results discourage the sharing of genetic data, which is becoming increasingly important for medical research. They go one step further and imply that people's possible hesitation to share their genetic information in public databases may actually play into Venter's hands.
Venter's own company, Human Longevity Inc., aims to build the world's most comprehensive private database on human genotypes and phenotypes. The vastness of this information stands to improve the accuracy of whole genome and microbiome sequencing for individuals—analyses that come at a hefty price tag. Today, Human Longevity Inc. will sequence your genome and perform a battery of other health-related tests at an entry cost of $4900, going up to $25,000. Venter initially agreed to comment for this article, but then could not be reached.
"The bigger issue is how do we understand and use genetic information and avoid harming people."
Opens Up Pandora's Box of Ethical Issues
Whether Venter's study is valid may not be as important as the Pandora's box of potential ethical and legal issues that it raises for future consideration. "I think this story is one along a continuum of stories we've had on the issue of identifiability based on genomic information in the past decade," said Amy McGuire, a biomedical ethics professor at Baylor College of Medicine. "It does raise really interesting and important questions about privacy, and socially, how we respond to these types of scientific advancements. A lot of our focus from a policy and ethics perspective is to protect privacy."
McGuire, who is also the Director of the Center for Medical Ethics and Health Policy at Baylor, added that while protecting privacy is very important, "the bigger issue is how do we understand and use genetic information and avoid harming people." While we've taken "baby steps," she said, towards enacting laws in the U.S. that fight genetic determinism—such as the Genetic Information and Nondiscrimination Act, which prohibits discrimination based on genetic information in health insurance and employment—some areas remain unprotected, such as for life insurance and disability.

J. Craig Venter showing slides from his recent study on facial prediction at the Summit Conference in Los Angeles on Nov. 3, 2017.
(Courtesy of Kira Peikoff)
Physical reconstructions like those in Venter's study could also be inappropriately used by law enforcement, said Leslie Francis, a law and philosophy professor at the University of Utah, who has written about the ethical and legal issues related to sharing genomic data.
"If [Venter's] findings, or findings like them, hold up, the implications would be significant," Francis said. Law enforcement is increasingly using DNA identification from genetic material left at crime scenes to weed out innocent and guilty suspects, she explained. This adds another potentially complicating layer.
"There is a shift here, from using DNA sequencing techniques to match other DNA samples—as when semen obtained from a rape victim is then matched (or not) with a cheek swab from a suspect—to using DNA sequencing results to predict observable characteristics," Francis said. She added that while the former necessitates having an actual DNA sample for a match, the latter can use DNA to pre-emptively (and perhaps inaccurately) narrow down suspects.
"My worry is that if this [the study's methodology] turns out to be sort-of accurate, people will think it is better than what it is," said Francis. "If law enforcement comes to rely on it, there will be a host of false positives and false negatives. And we'll face new questions, [such as] 'Which is worse? Picking an innocent as guilty, or failing to identify someone who is guilty?'"
Risking Privacy Involves a Tradeoff
When people voluntarily risk their own privacy, that involves a tradeoff, McGuire said. A 2014 study that she conducted among people who were very sick, or whose children were very sick, found that more than half were willing to share their health information, despite concerns about privacy, because they saw a big benefit in advancing research on their conditions.
"We've focused a lot of our policy attention on restricting access, but we don't have a system of accountability when there's a breach."
"To make leaps and bounds in medicine and genomics, we need to create a database of millions of people signing on to share their genetic and health information in order to improve research and clinical care," McGuire said. "They are going to risk their privacy, and we have a social obligation to protect them."
That also means "punishing bad actors," she continued. "We've focused a lot of our policy attention on restricting access, but we don't have a system of accountability when there's a breach."
Even though most people using genetic information have good intentions, the consequences if not are troubling. "All you need is one bad actor who decimates the trust in the system, and it has catastrophic consequences," she warned. That hasn't happened on a massive scale yet, and even if it did, some experts argue that obtaining the data is not the real risk; what is more concerning is hacking individuals' genetic information to be used against them, such as to prove someone is unfit for a particular job because of a genetic condition like Alzheimer's, or that a parent is unfit for custody because of a genetic disposition to mental illness.
Venter, in fact, told an audience at the recent Summit conference in Los Angeles that his new study's approach could not only predict someone's physical appearance from their DNA, but also some of their psychological traits, such as the propensity for an addictive personality. In the future, he said, it will be possible to predict even more about mental health from the genome.
What is most at risk on a massive scale, however, is not so much genetic information as demographic identifiers included in medical records, such as birth dates and social security numbers, said Francis, the law and philosophy professor. "The much more interesting and lucrative security breaches typically involve not people interested in genetic information per se, but people interested in the information in health records that you can't change."
Hospitals have been hacked for this kind of information, including an incident at the Veterans Administration in 2006, in which the laptop and external hard drive of an agency employee that contained unencrypted information on 26.5 million patients were stolen from the employee's house.
So, what can people do to protect themselves? "Don't share anything you wouldn't want the world to see," Francis said. "And don't click 'I agree' without actually reading privacy policies or terms and conditions. They may surprise you."
Scientists have known about and studied heart rate variability, or HRV, for a long time and, in recent years, monitors have come to market that can measure HRV accurately.
This episode is about a health metric you may not have heard of before: heart rate variability, or HRV. This refers to the small changes in the length of time between each of your heart beats.
Scientists have known about and studied HRV for a long time. In recent years, though, new monitors have come to market that can measure HRV accurately whenever you want.
Five months ago, I got interested in HRV as a more scientific approach to finding the lifestyle changes that work best for me as an individual. It's at the convergence of some important trends in health right now, such as health tech, precision health and the holistic approach in systems biology, which recognizes how interactions among different parts of the body are key to health.
But HRV is just one of many numbers worth paying attention to. For this episode of Making Sense of Science, I spoke with psychologist Dr. Leah Lagos; Dr. Jessilyn Dunn, assistant professor in biomedical engineering at Duke; and Jason Moore, the CEO of Spren and an app called Elite HRV. We talked about what HRV is, research on its benefits, how to measure it, whether it can be used to make improvements in health, and what researchers still need to learn about HRV.
*Talk to your doctor before trying anything discussed in this episode related to HRV and lifestyle changes to raise it.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Show notes
Spren - https://www.spren.com/
Elite HRV - https://elitehrv.com/
Jason Moore's Twitter - https://twitter.com/jasonmooreme?lang=en
Dr. Jessilyn Dunn's Twitter - https://twitter.com/drjessilyn?lang=en
Dr. Dunn's study on HRV, flu and common cold - https://jamanetwork.com/journals/jamanetworkopen/f...
Dr. Leah Lagos - https://drleahlagos.com/
Dr. Lagos on Star Talk - https://www.youtube.com/watch?v=jC2Q10SonV8
Research on HRV and intermittent fasting - https://pubmed.ncbi.nlm.nih.gov/33859841/
Research on HRV and Mediterranean diet - https://medicalxpress.com/news/2010-06-twin-medite...:~:text=Using%20data%20from%20the%20Emory,eating%20a%20Western%2Dtype%20diet
Devices for HRV biofeedback - https://elitehrv.com/heart-variability-monitors-an...
Benefits of HRV biofeedback - https://pubmed.ncbi.nlm.nih.gov/32385728/
HRV and cognitive performance - https://www.frontiersin.org/articles/10.3389/fnins...
HRV and emotional regulation - https://pubmed.ncbi.nlm.nih.gov/36030986/
Fortune article on HRV - https://fortune.com/well/2022/12/26/heart-rate-var...
Peanut allergies affect about a million children in the U.S., and most never outgrow them. Luckily, some promising remedies are in the works.
Ever since he was a baby, Sharon Wong’s son Brandon suffered from rashes, prolonged respiratory issues and vomiting. In 2006, as a young child, he was diagnosed with a severe peanut allergy.
"My son had a history of reacting to traces of peanuts in the air or in food,” says Wong, a food allergy advocate who runs a blog focusing on nut free recipes, cooking techniques and food allergy awareness. “Any participation in school activities, social events, or travel with his peanut allergy required a lot of preparation.”
Peanut allergies affect around a million children in the U.S. Most never outgrow the condition. The problem occurs when the immune system mistakenly views the proteins in peanuts as a threat and releases chemicals to counteract it. This can lead to digestive problems, hives and shortness of breath. For some, like Wong’s son, even exposure to trace amounts of peanuts could be life threatening. They go into anaphylactic shock and need to take a shot of adrenaline as soon as possible.
Typically, people with peanut allergies try to completely avoid them and carry an adrenaline autoinjector like an EpiPen in case of emergencies. This constant vigilance is very stressful, particularly for parents with young children.
“The search for a peanut allergy ‘cure’ has been a vigorous one,” says Claudia Gray, a pediatrician and allergist at Vincent Pallotti Hospital in Cape Town, South Africa. The closest thing to a solution so far, she says, is the process of desensitization, which exposes the patient to gradually increasing doses of peanut allergen to build up a tolerance. The most common type of desensitization is oral immunotherapy, where patients ingest small quantities of peanut powder. It has been effective but there is a risk of anaphylaxis since it involves swallowing the allergen.
"By the end of the trial, my son tolerated approximately 1.5 peanuts," Sharon Wong says.
DBV Technologies, a company based in Montrouge, France has created a skin patch to address this problem. The Viaskin Patch contains a much lower amount of peanut allergen than oral immunotherapy and delivers it through the skin to slowly increase tolerance. This decreases the risk of anaphylaxis.
Wong heard about the peanut patch and wanted her son to take part in an early phase 2 trial for 4-to-11-year-olds.
“We felt that participating in DBV’s peanut patch trial would give him the best chance at desensitization or at least increase his tolerance from a speck of peanut to a peanut,” Wong says. “The daily routine was quite simple, remove the old patch and then apply a new one. By the end of the trial, he tolerated approximately 1.5 peanuts.”
How it works
For DBV Technologies, it all began when pediatric gastroenterologist Pierre-Henri Benhamou teamed up with fellow professor of gastroenterology Christopher Dupont and his brother, engineer Bertrand Dupont. Together they created a more effective skin patch to detect when babies have allergies to cow's milk. Then they realized that the patch could actually be used to treat allergies by promoting tolerance. They decided to focus on peanut allergies first as the more dangerous.
The Viaskin patch utilizes the fact that the skin can promote tolerance to external stimuli. The skin is the body’s first defense. Controlling the extent of the immune response is crucial for the skin. So it has defense mechanisms against external stimuli and can promote tolerance.
The patch consists of an adhesive foam ring with a plastic film on top. A small amount of peanut protein is placed in the center. The adhesive ring is attached to the back of the patient's body. The peanut protein sits above the skin but does not directly touch it. As the patient sweats, water droplets on the inside of the film dissolve the peanut protein, which is then absorbed into the skin.
The peanut protein is then captured by skin cells called Langerhans cells. They play an important role in getting the immune system to tolerate certain external stimuli. Langerhans cells take the peanut protein to lymph nodes which activate T regulatory cells. T regulatory cells suppress the allergic response.
A different patch is applied to the skin every day to increase tolerance. It’s both easy to use and convenient.
“The DBV approach uses much smaller amounts than oral immunotherapy and works through the skin significantly reducing the risk of allergic reactions,” says Edwin H. Kim, the division chief of Pediatric Allergy and Immunology at the University of North Carolina, U.S., and one of the principal investigators of Viaskin’s clinical trials. “By not going through the mouth, the patch also avoids the taste and texture issues. Finally, the ability to apply a patch and immediately go about your day may be very attractive to very busy patients and families.”
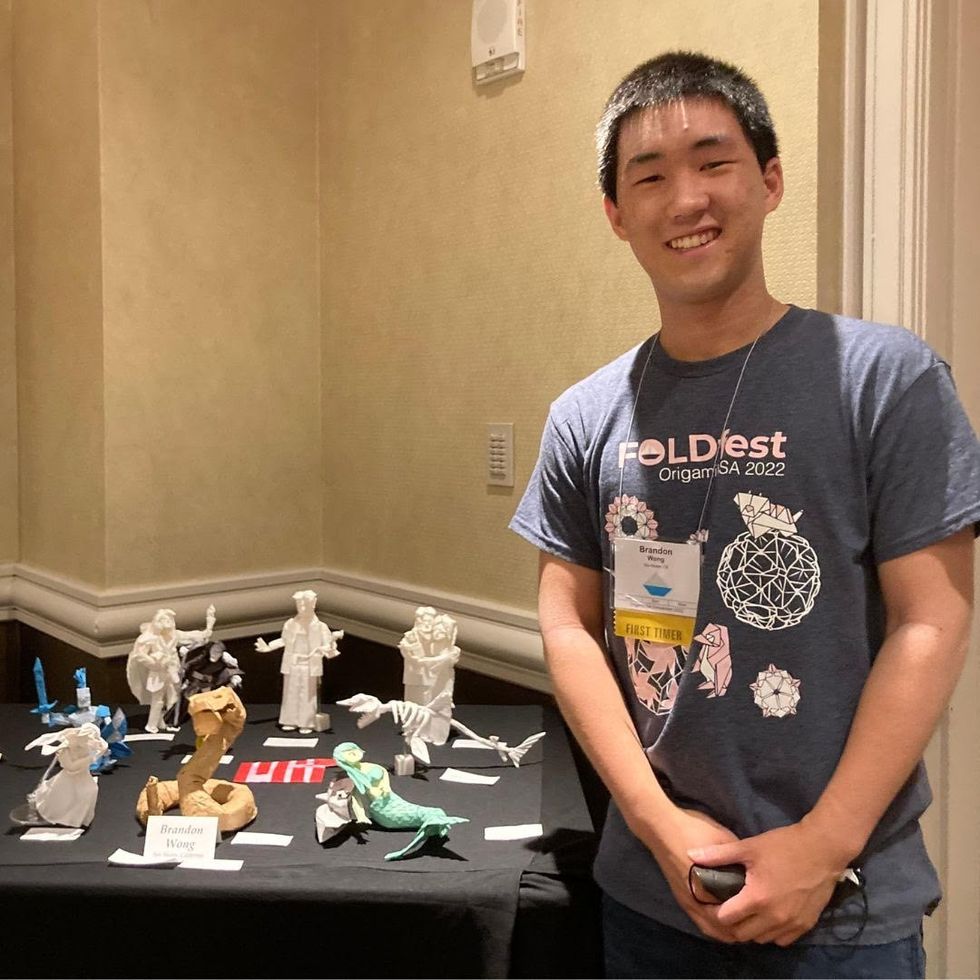
Brandon Wong displaying origami figures he folded at an Origami Convention in 2022
Sharon Wong
Clinical trials
Results from DBV's phase 3 trial in children ages 1 to 3 show its potential. For a positive result, patients who could not tolerate 10 milligrams or less of peanut protein had to be able to manage 300 mg or more after 12 months. Toddlers who could already tolerate more than 10 mg needed to be able to manage 1000 mg or more. In the end, 67 percent of subjects using the Viaskin patch met the target as compared to 33 percent of patients taking the placebo dose.
“The Viaskin peanut patch has been studied in several clinical trials to date with promising results,” says Suzanne M. Barshow, assistant professor of medicine in allergy and asthma research at Stanford University School of Medicine in the U.S. “The data shows that it is safe and well-tolerated. Compared to oral immunotherapy, treatment with the patch results in fewer side effects but appears to be less effective in achieving desensitization.”
The primary reason the patch is less potent is that oral immunotherapy uses a larger amount of the allergen. Additionally, absorption of the peanut protein into the skin could be erratic.
Gray also highlights that there is some tradeoff between risk and efficacy.
“The peanut patch is an exciting advance but not as effective as the oral route,” Gray says. “For those patients who are very sensitive to orally ingested peanut in oral immunotherapy or have an aversion to oral peanut, it has a use. So, essentially, the form of immunotherapy will have to be tailored to each patient.” Having different forms such as the Viaskin patch which is applied to the skin or pills that patients can swallow or dissolve under the tongue is helpful.
The hope is that the patch’s efficacy will increase over time. The team is currently running a follow-up trial, where the same patients continue using the patch.
“It is a very important study to show whether the benefit achieved after 12 months on the patch stays stable or hopefully continues to grow with longer duration,” says Kim, who is an investigator in this follow-up trial.
"My son now attends university in Massachusetts, lives on-campus, and eats dorm food. He has so much more freedom," Wong says.
The team is further ahead in the phase 3 follow-up trial for 4-to-11-year-olds. The initial phase 3 trial was not as successful as the trial for kids between one and three. The patch enabled patients to tolerate more peanuts but there was not a significant enough difference compared to the placebo group to be definitive. The follow-up trial showed greater potency. It suggests that the longer patients are on the patch, the stronger its effects.
They’re also testing if making the patch bigger, changing the shape and extending the minimum time it’s worn can improve its benefits in a trial for a new group of 4-to-11 year-olds.
The future
DBV Technologies is using the skin patch to treat cow’s milk allergies in children ages 1 to 17. They’re currently in phase 2 trials.
As for the peanut allergy trials in toddlers, the hope is to see more efficacy soon.
For Wong’s son who took part in the earlier phase 2 trial for 4-to-11-year-olds, the patch has transformed his life.
“My son continues to maintain his peanut tolerance and is not affected by peanut dust in the air or cross-contact,” Wong says. ”He attends university in Massachusetts, lives on-campus, and eats dorm food. He still carries an EpiPen but has so much more freedom than before his clinical trial. We will always be grateful.”

