Is Sex for Reproduction About to Become Extinct?
A healthy fetus in utero at 22 weeks.
There are lots of great reasons we humans have sex. We mostly do it to pair bond, realize our primal urges, and feel good. Once in a while, we also do it to make babies. As the coming genetic revolution plays out, we'll still have sex for most of the same reasons we do today. But we'll increasingly not do it to procreate.
Protecting children from harm is one of the core responsibilities of parenting.
Most parents go to great lengths to protect their children from real and imagined harms. This begins with taking prenatal vitamins during pregnancy and extends to having children immunized and protected from exposures to various diseases and dangers. Most of us look askance for good reason at mothers who abuse controlled substances during their pregnancies or parents who choose to not immunize their children. Protecting children from harm is one of the core responsibilities of parenting.
In the United States today, up to two percent of babies are estimated to be born with rare genetic diseases caused by single gene mutations. Sickle cell disease, Tay-Sachs, and Huntington's disease are among the more well-known examples of these, but the list runs to the thousands. Many babies born with these disorders suffer terribly, some die young, and nearly all spend big chunks of their lives struggling through the medical system.
Increasingly, however, many of these single-gene mutation diseases and other chromosomal disorders like Down syndrome are being identified in non-invasive prenatal tests performed on expectant mothers at the end of their first trimester of pregnancy. Knowing the hardship that children born with these types of disorders will likely face, majorities of these women in countries around the world are choosing to terminate pregnancies once these diagnoses have been made. Whatever the justification and whatever anyone's views on the morality of abortion, these decisions are inherently excruciating.
A much smaller number of prospective mothers, however, are today getting this same information about their potential future children before their pregnancies even begin. By undergoing both in vitro fertilization (IVF) and preimplantation genetic testing (PGT), these women are able to know which of the eggs that have been surgically extracted from them and fertilized with their partner or donor's sperm will carry the dangerous mutations. The in vitro embryos with these disorders are simply not implanted in the expectant mother's womb.
It would be monstrous to assert that an existing person with a deadly disease has any less right to thrive than anyone else. But it would also be hard to make a case that parents should affirmatively choose to implant embryos carrying such a disease if given the option. If prospective parents are already today choosing not to implant certain embryos based on our preliminary understanding of disease risk, what will happen when this embryo selection is based on far more information than just a few thousand single gene mutation diseases?
Our ability and willingness to make genetic alterations to our future children will grow over time along with our knowledge and technological ability.
When the first human genome was sequenced in 2003, the race to uncover the mysteries of human genetics had only just begun. Although we still know very little about our genetics relative to the complexity of the genome and even less compared to the broader ecosystem of our biology, the progress toward greater understanding is astounding. Today, the number of single gene mutation diseases and relatively simple genetic traits that can be predicted meaningfully from genetic data alone is already significant.
In the not-distant future, this list will grow to include complex diseases and disease propensities, percentage probabilities of living a long and healthy life, and increasingly the genetic component of complex human attributes like height, IQ, and personality style. This predictive power of genetic analysis will funnel straight into our fertility clinics where prospective parents choosing embryos will be making ever more consequential decisions about the genetic components of the future lives, health, and capabilities of their children.
Our understanding of what the genes extracted from early stage pre-implanted embryos are telling us will be only one of the rocket boosters driving assisted reproduction forward. Another will be the ability to induce adult cells like skin and nucleated blood cells into stem cells and then turn those stem cells into egg progenitor cells and then ultimately eggs. This will not only eliminate the need for hormone treatments and surgery to extract human eggs but also make it easy and cheap to generate an unlimited number of eggs from a given woman.
The average woman has around fifteen eggs extracted during IVF but imagine what generating a thousand eggs will do to the range of possibilities that could be realized through pre-implantation embryo selection. Each of these thousand eggs would be the natural offspring of the two parents, but the variation between them would make it possible to choose the ones with the strongest expression of the genetic component of a particular desired trait – like those with the highest possible genetic IQ potential.
Another rocket booster will be the application of gene editing technologies like CRISPR to edit the genomes of pre-implanted embryos or of the sperm and eggs used to create them. Just this week, Chinese researchers announced they had used CRISPR to edit the CCR5 gene in the pre-implanted embryos of a pair of Chinese twins to make them immune to HIV, the first ever case of gene editing humans and a harbinger of our genetically engineered future. The astounding complexity of the human genome will put limits on our ability to safely make too many simultaneous genetic changes to human embryos, but our ability and willingness to make these types of alterations to our future children will grow over time along with our knowledge and technological ability.
With so much at stake, prospective parents will increasingly have a stark choice when determining how to conceive their children. If they go the traditional route of sex, they will experience both the benign wisdom and unfathomable cruelty of nature. If they use IVF and increasingly informed embryo selection, they will eliminate most single gene mutation diseases and likely increase their children's chances of living a longer and healthier life with more opportunity than their unenhanced peers. But the optimizing parents could also set up their children for misery if these children don't particularly enjoy what they have been optimized to become or see themselves as some type of freakish consumer product with emotions.
Conceiving though sex will come to be seen more and more like not immunizing your children is today, a perfectly natural choice that comes with a significant potential risk and expense.
But although there will be pros and cons on each side, the fight between conception through good old-fashioned sex and conception in the lab will ultimately not be fair. Differences and competition within and between societies will pressure parents and societies to adopt ever more aggressive forms of reproductive technology if they believe doing so will open possibilities and create opportunities for the next generations rather than close them.
Conception through sex will remain as useful as it has always been but lab conception will only get more advantageous. Over time, only zealots will choose to roll the dice of their future children's health and well-being rather than invest, like parents always have, in protecting their children from harm and helping optimize their life potential. Conceiving though sex will come to be seen more and more like not immunizing your children is today, a perfectly natural choice that comes with a significant potential risk and expense to yourself, your children, and your community.
As this future plays out, the genetics and assisted reproduction revolutions will raise enormous, thorny, and massively consequential questions about how we value and invest in diversity, equality, and our own essential humanity – questions we aren't remotely prepared to answer. But these revolutions are coming sooner than most of us understand or are prepared for so we had better get ready.
Because where this trail is ultimately heading goes well beyond sex and toward a fundamental transformation of our evolutionary process as a species – and that should be everybody's business.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
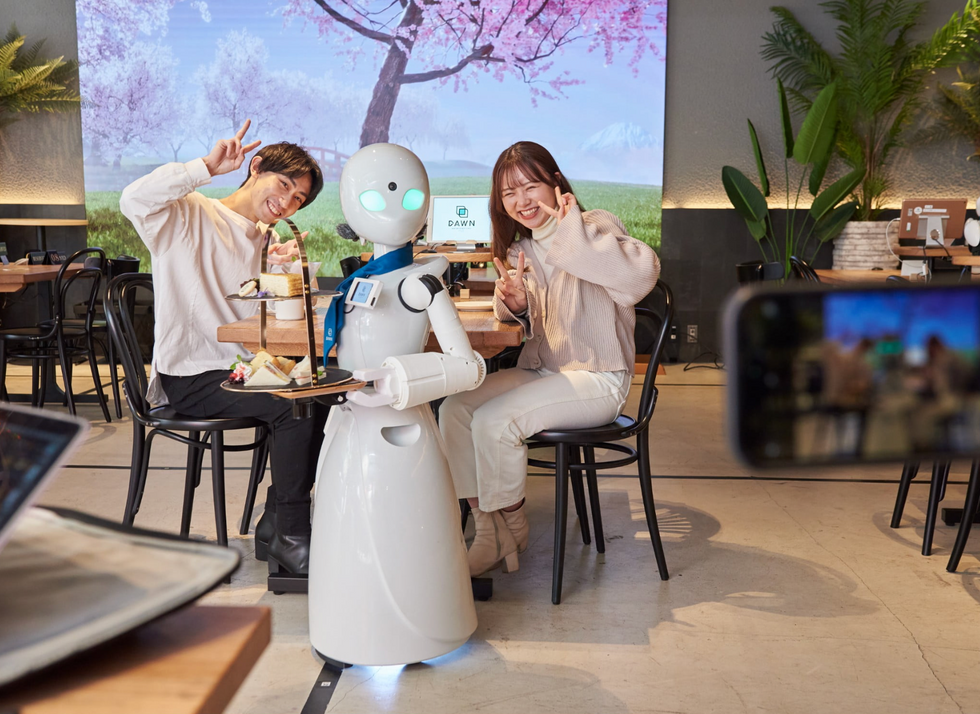
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
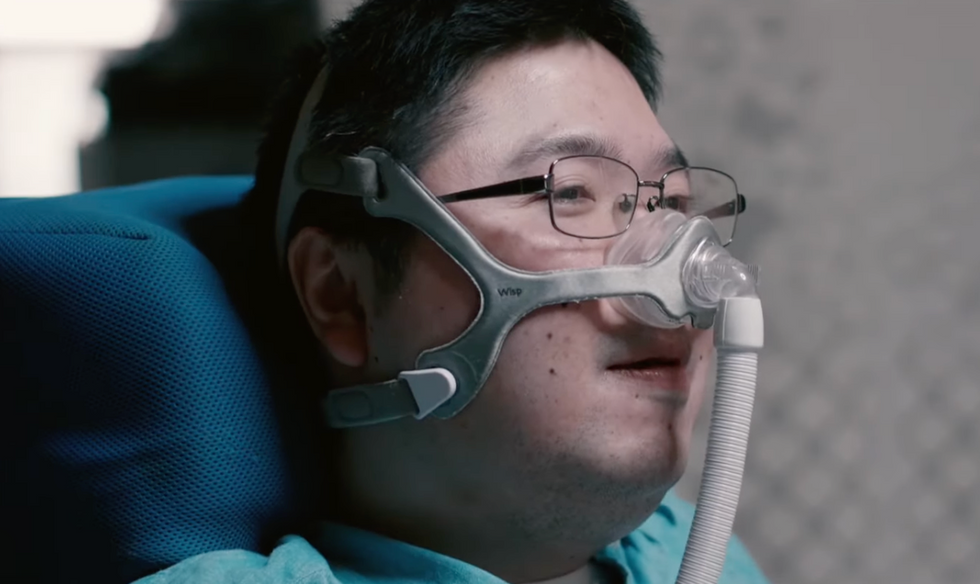
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

