Our Genetically Engineered Future Is Closer Than You Think

Study of the DNA double helix will lead to transformative medical care and increasingly urgent questions about how to responsibly handle genetic engineering technology.
The news last November that a rogue Chinese scientist had genetically altered the embryos of a pair of Chinese twins shocked the world. But although this use of advanced technology to change the human gene pool was premature, it was a harbinger of how genetic science will alter our healthcare, the way we make babies, the nature of the babies we make, and, ultimately, our sense of who and what we are as a species.
The healthcare applications of the genetics revolution are merely stations along the way to the ultimate destination.
But while the genetics revolution has already begun, we aren't prepared to handle these Promethean technologies responsibly.
By identifying the structure of DNA in the 1950s, Watson, Crick, Wilkins, and Franklin showed that the book of life was written in the DNA double helix. When the human genome project was completed in 2003, we saw how this book of human life could be transcribed. Painstaking research paired with advanced computational algorithms then showed what increasing numbers of genes do and how the genetic book of life can be read.
Now, with the advent of precision gene editing tools like CRISPR, we are seeing that the book of life -- and all biology -- can be re-written. Biology is being recognized as another form of readable, writable, and hackable information technology with we humans as the coders.
The impact of this transformation is being first experienced in our healthcare. Gene therapies including those extracting, re-engineering, then reintroducing a person's own cells enhanced into cancer-fighting supercells are already performing miracles in clinical trials. Thousands of applications have already been submitted to regulators across the globe for trials using gene therapies to address a host of other diseases.
Recently, the first gene editing of cells inside a person's body was deployed to treat the genetically relatively simple metabolic disorder Hunter syndrome, with many more applications to come. These new approaches are only the very first steps in our shift from the current system of generalized medicine based on population averages to precision medicine based on each patient's individual biology to predictive medicine based on AI-generated estimations of a person's future health state.

Jamie Metzl's groundbreaking new book, Hacking Darwin: Genetic Engineering and the Future of Humanity, explores how the genetic revolution is transforming our healthcare, the way we make babies, and the nature of and babies we make, what this means for each of us, and what we must all do now to prepare for what's coming.
This shift in our healthcare will ensure that millions and then billions of people will have their genomes sequenced as the foundation of their treatment. Big data analytics will then be used to compare at scale people's genotypes (what their genes say) to their phenotypes (how those genes are expressed over the course of their lives).
These massive datasets of genetic and life information will then make it possible to go far beyond the simple genetic analysis of today and to understand far more complex human diseases and traits influenced by hundreds or thousands of genes. Our understanding of this complex genetic system within the vaster ecosystem of our bodies and the environment around us will transform healthcare for the better and help us cure terrible diseases that have plagued our ancestors for millennia.
But as revolutionary as this challenge will be for medicine, the healthcare applications of the genetics revolution are merely stations along the way to the ultimate destination – a deep and fundamental transformation of our evolutionary trajectory as a species.
A first inkling of where we are heading can be seen in the direct-to-consumer genetic testing industry. Many people around the world have now sent their cheek swabs to companies like 23andMe for analysis. The information that comes back can tell people a lot about relatively simple genetic traits like carrier status for single gene mutation diseases, eye color, or whether they hate the taste of cilantro, but the information about complex traits like athletic predisposition, intelligence, or personality style today being shared by some of these companies is wildly misleading.
This will not always be the case. As the genetic and health data pools grow, analysis of large numbers of sequenced genomes will make it possible to apply big data analytics to predict some very complex genetic disease risks and the genetic components of traits like height, IQ, temperament, and personality style with increasing accuracy. This process, called "polygenic scoring," is already being offered in beta stage by a few companies and will become an ever bigger part of our lives going forward.
The most profound application of all this will be in our baby-making. Before making a decision about which of the fertilized eggs to implant, women undergoing in vitro fertilization can today elect to have a small number of cells extracted from their pre-implanted embryos and sequenced. With current technology, this can be used to screen for single-gene mutation diseases and other relatively simple disorders. Polygenic scoring, however, will soon make it possible to screen these early stage pre-implanted embryos to assess their risk of complex genetic diseases and even to make predictions about the heritable parts of complex human traits. The most intimate elements of being human will start feeling like high-pressure choices needing to be made by parents.
The limit of our imagination will become the most significant barrier to our recasting biology.
Adult stem cell technologies will then likely make it possible to generate hundreds or thousands of a woman's own eggs from her blood sample or skin graft. This would blow open the doors of reproductive possibility and allow parents to choose embryos with exceptional potential capabilities from a much larger set of options.
The complexity of human biology will place some limits to the extent of possible gene edits that might be made to these embryos, but all of biology, including our own, is extremely flexible. How else could all the diversity of life have emerged from a single cell nearly four billion years ago? The limit of our imagination will become the most significant barrier to our recasting biology.
But while we humans are gaining the powers of the gods, we aren't at all ready to use them.
The same tools that will help cure our worst afflictions, save our children, help us live longer, healthier, more robust lives will also open the door to potential abuses. Prospective parents with the best of intentions or governments with lax regulatory structures or aggressive ideas of how population-wide genetic engineering might be used to enhance national competitiveness or achieve some other goal could propel us into a genetic arms race that could undermine our essential diversity, dangerously divide societies, lead to dangerous, destabilizing, and potentially even deadly conflicts between us, and threaten our very humanity.
But while the advance of genetic technologies is inevitable, how it plays out is anything but. If we don't want the genetic revolution to undermine our species or lead to grave conflicts between genetic haves and have nots or between societies opting in and those opting out, now is the time when we need to make smart decisions based on our individual and collective best values. Although the technology driving the genetic revolution is new, the value systems we will need to optimize the benefits and minimize the harms of this massive transformation are ones we have been developing for thousands of years.
And while some very smart and well-intentioned scientists have been meeting to explore what comes next, it won't be enough for a few of even our wisest prophets to make decisions about the future of our species that will impact everyone. We'll also need smart regulations on both the national and international levels.
Every country will need to have its own regulatory guidelines for human genetic engineering based on both international best practices and the country's unique traditions and values. Because we are all one species, however, we will also ultimately need to develop guidelines that can apply to all of us.
As a first step toward making this possible, we must urgently launch a global, species-wide education effort and inclusive dialogue on the future of human genetic engineering that can eventually inform global norms that will need to underpin international regulations. This process will not be easy, but the alternative of an unregulated genetic arms race would be far worse.
The overlapping genomics and AI revolutions may seem like distant science fiction but are closer than you think. Far sooner than most people recognize, the inherent benefits of these technologies and competition between us will spark rapid adoption. Before that spark ignites, we have a brief moment to come together as a species like we never have before to articulate and translate into action the future we jointly envision. The north star of our best shared values can help us navigate the almost unimaginable opportunities and very real challenges that lie ahead.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
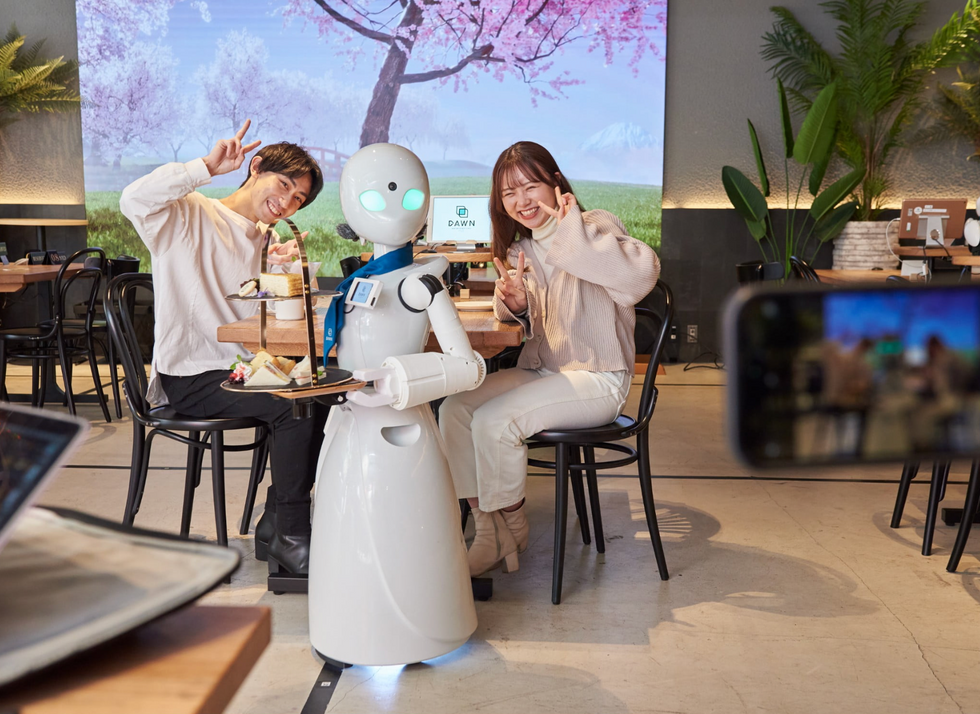
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
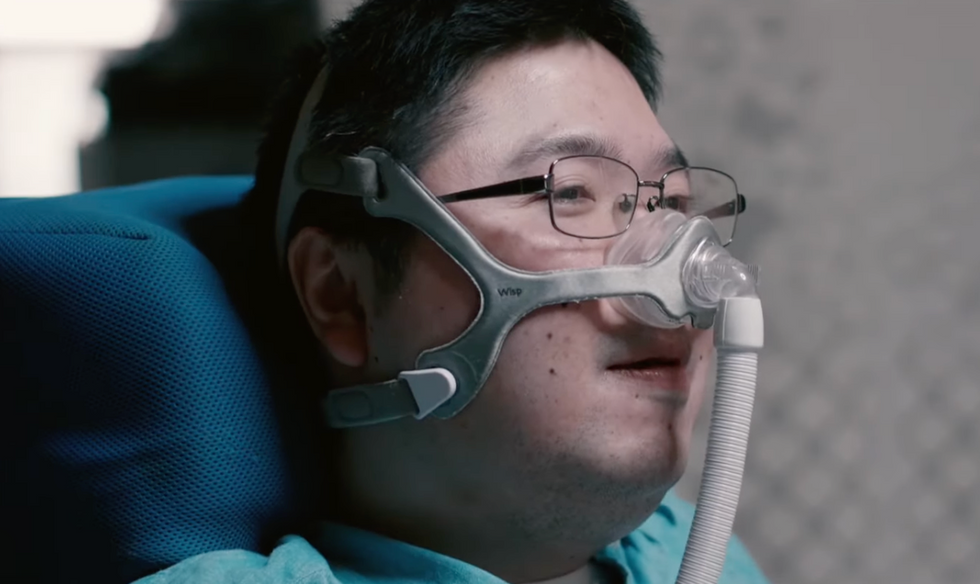
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

