Parkinson’s Disease Destroyed My Life. Then I Tried Deep Brain Stimulation.

Anne, Stan, and grandson Louie during vacation in Mexico, 2019. INSET: Anne post-op in 2017.
[Editor's Note: On June 6, 2017, Anne Shabason, an artist, hospice educator, and mother of two from Bolton, Ontario, a small town about 30 miles outside of Toronto, underwent Deep Brain Stimulation (DBS) to treat her Parkinson's disease. The FDA approved DBS for Parkinson's disease in 2002. Although it's shown to be safe and effective, agreeing to invasive brain surgery is no easy decision, even when you have your family and one of North America's premier neurosurgeons at your side.
Here, with support from Stan, her husband of the past 40 years, Anne talks about her life before Parkinson's, what the disease took away, and what she got back because of DBS. As told to writer Heather R. Johnson.]
I was an artist.
I worked in mixed media, Papier-mâché, and collage, inspired by dreams, birds, mystery. I had gallery shows and participated in studio tours.
Educated in thanatology, I worked in hospice care as a volunteer and education director for Hospice Caledon, an organization that supports people facing life-limiting illness and grief.
I trained volunteers who helped people through their transition.
Parkinson's disease changed all that.
My hands and my head were not coordinating, so it was impossible to do my art.
It started as a twitch in my leg. During a hospice workshop, my right leg started vibrating in a way I hadn't experienced before. I told a friend, "This can't be good."
Over the next year, my right foot vibrated more and more. I could not sleep well. In my dreams people lurked in corners, in dark places, and behind castle doors. I knew they were there and couldn't avoid the ambush. I shrieked and woke everyone in the house.
An anxiety attack—something I had also never experienced before—came next.
During a class I was teaching, my mouth got so dry, I couldn't speak. I stood in front of the class for three or four minutes, unable to continue. I pushed through and finished the class. That's when I realized this was more than jiggling legs.
That's when I went to see a doctor.
A Diagnosis
My first doctor, when I suggested it might be Parkinson's, didn't believe me. She sent me to a neurologist who told me I had to meditate more and calm myself.
A friend from hospice told me to phone the Toronto Western Hospital Movement Disorders Clinic. In January 2010, I was diagnosed with Parkinson's disease.
The doctor, a fellow, got all my stats and asked a lot of questions. He was so excited he knew what it was, he exclaimed, "You've got Parkinson's!" like it was the best thing ever. I must say, that wasn't the best news, but at least I finally had a diagnosis.
I could choose whether to take medication or not. The doctor said, "If Parkinson's is compromising your lifestyle, you should consider taking levodopa."
"Well I can't run my classes, I can't do my art, so it's compromising me," I said. And my health was going downhill. The shaking—my whole body moved—sleeping was horrible. Two to four hours max a night was usual. I had terrible anxiety and panic attacks and had to quit work.
So I started taking levodopa. It's taken in a four-hour cycle, but the medication didn't last the full time. I developed dyskenisia, a side effect of the medication that made me experience uncontrolled, involuntary movements. I was edgy, irritable, and focused on my watch like a drug addict. I'd lie on the couch, feel crummy and tired, and wait.
The medication cycle restricted where I could go. Fearing the "off" period, I avoided interaction with lifelong friends, which increased my feeling of social isolation. They would come over and cook with me and read to me sometimes, and that was fine, as long as it was during an "on" period.
There was incontinence, constipation, and fatigue.
I lost fine motor skills, like writing. And painting. My hands and my head were not coordinating, so it was impossible to do my art.
It was a terrible time.
The worst symptoms—what pushed me to consider DBS—were the symptoms no one could see. The anxiety and depression were so bad, the sleeplessness, not eating.
I projected a lot of my discomforts onto Stan. I reacted so badly to him. I actually separated from him briefly on two separate occasions and lived in a separate space—a self-imposed isolation. There wasn't anything he could do to help me really except sit back and watch.
I tried alternative therapies—a naturopath, an osteopath, a reflexologist and a Chinese medicine practitioner—but nothing seemed to help.
I felt like I was dying. Certain parts of my life were being taken away from me. I was a perfectionist, and I felt imperfect. It was a horrible feeling, to not be in control of myself.
The DBS Decision
I was familiar with DBS, a procedure that involves a neurosurgeon drilling small holes into your skull and implanting electrical leads deep in your brain to modify neural activity, reducing involuntary movements.
But I was convinced I'd never do it. I was brought up in a family that believed 'doctors make you sick and hospitals kill you.'
I worried the room wouldn't be sterile. Someone's cutting into your brain, you don't know what's going to happen. They're putting things in your body. I didn't want to risk possible infection.
And my doctor said he couldn't promise he would actually do the operation. It might be a fellow, but he'd be in the background in case anything went wrong. I wasn't comfortable with that arrangement.
When filmmakers Taryn Southern and Elena Gaby decided to make a documentary about people whose lives were changed by cutting-edge brain implants--and I agreed to participate—my doctor said he would for sure do the operation. They couldn't risk anything happening on the operating table on camera, so most of my fears went away.
My family supported the decision. My mother had trigeminal neuralgia, which is a very painful facial condition. She also had a stroke and what we now believe to be Parkinson's. My father, a retired dentist, managed her care and didn't give her the opportunity to see a specialist.
I felt them running the knife across my scalp, and drilling two holes in my head, but only as pressure, not pain.
When we were talking about DBS, my son, Joseph, said, "How can you not do this, for the sake of your family? Because if you don't, you'll end up like Grandma, who, for the last few years of her life, just lay on a couch because she didn't get any kind of outside help. If you even have a chance to improve your life or give yourself five extra years, why wouldn't you do that, for our sake? Are we not worth that?"
That talk really affected me, and I realized I had to try. Even though it was difficult, I had to be brave for my family.
Surgery, Recovery, and Tweaking
You have to be awake for part of the procedure—I was awake enough that my subconscious could hear, because they had to know how far to insert the electrodes. DBS targets the troublemaking areas of the brain. There's a one millimeter difference between success and failure.
I felt them running the knife across my scalp, and drilling two holes in my head, but only as pressure, not pain.
Once they were inside, they asked me to move parts of my body to see whether the right neurons were activated.
They put me to sleep to put a battery-powered neurostimulator in my chest. A wire that runs behind my ear and down my neck connects the electrodes in my brain to the battery pack. The neurostimulator creates electric pulses 24 hours a day.
I was moving around almost immediately after surgery. Recovery from the stitches took a few weeks, but everything else took a lot longer.
I couldn't read. My motor skills were still impaired, and my brain and my hands weren't yet linked up. I needed the device to be programmed and tweaked. Until that happened, I needed help.
The depression and anxiety, though, went away almost immediately. From that perspective, it was like I never had Parkinson's. I was so happy.
When they calibrated the electrodes, they adjusted how much electrical current goes to any one of four contact points on the left and right sides of the brain. If they increased it too much, a leg would start shaking, a foot would start cramping, or my tongue would feel thicker. It took a while to get it calibrated correctly to control the symptoms.
First it was five sessions in five weeks, then once a month, then every three months. Now I visit every six months. As the disease progresses, they have the ability to keep making adjustments. (DBS controls the symptoms, but it doesn't cure the disease.)
Once they got the calibration right, my motor skills improved. I could walk without shuffling. My muscles weren't stiff and aching, and the dyskinesia disappeared. But if I turn off the device, my symptoms return almost immediately.
Some days I have more fatigue than others, and sometimes my brain doesn't click. And my voice got softer – that's a common side effect of this operation. But I'm doing so much better than before.
I have a quality of life I didn't have before. Before COVID-19 hit, Stan and I traveled, went to concerts, movies, galleries, and spent time with our growing family.
I cut back the levodopa from seven-and-a-half pills a day to two-and-a-half. I often forget to take my medication until I realize I'm feeling tired or anxious.
Best of all, my motivation and creative ability have clicked in.
I am an artist—again.
I'm painting every day. It's what is keeping me sane. It's my saving grace.
I'm not perfect. But I am Anne. Again.
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained
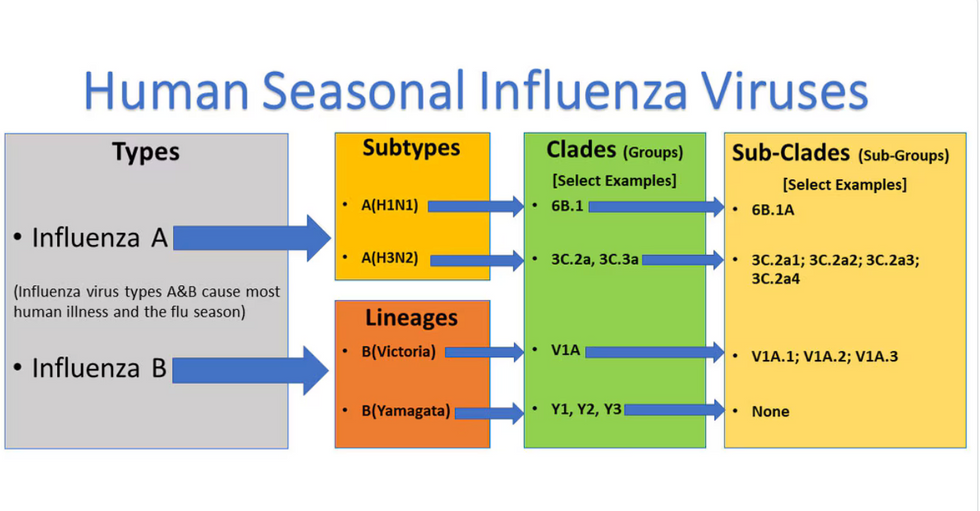
Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.


