This Revolutionary Medical Breakthrough Is Not a Treatment or a Cure

A doctor examines a patient to determine the cause of her illness.
What is a disease? This seemingly abstract and theoretical question is actually among the most practical questions in all of biomedicine. How patients are diagnosed, treated, managed and excused from various social and moral obligations hinges on the answer that is given. So do issues of how research is done and health care paid for. The question is also becoming one of the most problematic issues that those in health care will face in the next decade.
"The revolution in our understanding of the human genome, molecular biology, and genetics is creating a huge--if little acknowledged--shift in the understanding of what a disease is."
That is because the current conception of disease is undergoing a revolutionary change, fueled by progress in genetics and molecular biology. The consequences of this shift in the definition of disease promise to be as impactful as any other advance in biomedicine has ever been, which is admittedly saying a lot for what is in essence a conceptual change rather than one based on an empirical scientific advance.
For a long time, disease was defined by patient reports of feeling sick. It was not until the twentieth century that a shift occurred away from subjective reports of clusters of symptoms to defining diseases in terms of physiological states. Doctors began to realize that not all symptoms of fever represented the presence of the same disease. Flu got distinguished from malaria. Diseases such as hypertension, osteoporosis, cancer, lipidemia, silent myocardial infarction, retinopathy, blood clots and many others were recognized as not producing any or slight symptoms until suddenly the patient had a stroke or died.
The ability to assess both biology and biochemistry and to predict the consequences of subclinical pathological processes caused a distinction to be made between illness—what a person experiences—and disease—an underlying pathological process with a predictable course. Some conditions, such as Gulf War Syndrome, PTSD, many mental illnesses and fibromyalgia, remain controversial because no underlying pathological process has been found that correlates with them—a landmark criterion for diagnosing disease throughout most of the last century.
"Diseases for which no relationship had ever been posited are being lumped together due to common biochemical causal pathways...that are amenable to the same curative intervention."
The revolution in our understanding of the human genome, molecular biology, and genetics is creating a huge--if little acknowledged--shift in the understanding of what a disease is. A better understanding of the genetic and molecular roots of pathophysiology is leading to the reclassification of many familiar diseases. The test of disease is now not the pathophysiology but the presence of a gene, set of genes or molecular pathway that causes pathophysiology. Just as fever was differentiated into a multitude of diseases in the last century, cancer, cognitive impairment, addiction and many other diseases are being broken or split into many subkinds. And other diseases for which no relationship had ever been posited are being lumped together due to common biochemical causal pathways or the presence of similar dangerous biochemical products that are amenable to the same curative intervention, no matter how disparate the patients' symptoms or organic pathologies might appear.
We used to differentiate ovarian and breast cancers. Now we are thinking of them as outcomes of the same mutations in certain genes in the BRCA regions. They may eventually lump together as BRCA disease.
Other diseases such as familial amyloid polyneuropathy (FAP) which causes polyneuropathy and autonomic dysfunction are being split apart into new types or kinds. The disease is the product of mutations in the transthyretin gene. It was thought to be an autosomal dominant disease with symptomatic onset between 20-40 years of age. However, as genetic testing has improved, it has become clear that FAP's traditional clinical presentation represents a relatively small portion of those with FAP. Many patients with mutations in transthyretin — even mutations commonly seen in traditional FAP patients — do not fit the common clinical presentation. As the mutations begin to be understood, some people that were previously thought to have other polyneuropathies, such as chronic inflammatory demyelinating neuropathy, are now being rediagnosed with newly discovered variants of FAP.
"We are at the start of a major conceptual shift in how we organize the world of disease, and for that matter, health promotion."
Genome-wide association studies are beginning to find many links between diseases not thought to have any connection or association. For example some forms of diabetes, rheumatoid arthritis and thyroid disease may be the products of a small family of genetic mutations.
So why is this shift toward a genetic and molecular diagnostics likely to shake up medicine? One obvious way is that research projects may propose to recruit subjects not according to current standards of disease but on the basis of common genetic mutations or similar errors in biochemical pathways. It won't matter in a future study if subjects in a trial have what today might be termed nicotine addiction or Parkinsonism. If the molecular pathways producing the pathology are the same, then both groups might well wind up in the same trial of a drug.
In addition, what today look like common maladies—pancreatic cancer, severe depression, or acne, for example, could wind up being subdivided into so many highly differentiated versions of these conditions that each must be treated as what we now classify as a rare or ultra-rare disease. Unique biochemical markers or genetic messages may see many diseases broken into a huge number of distinct individual disease entities.
Patients may find that common genetic pathways or multiple effects from a single gene may create new alliances for fund-raising and advocacy. Groups fighting to cure mental and physical illnesses may wind up forgetting about their outward differences in the effort to alter genes or attack common protein markers.
Disease classification appears stable to us—until it isn't. And we are at the start of a major conceptual shift in how we organize the world of disease, and for that matter, health promotion. Classic reductionism, the view that all observable biological phenomena can be explained in terms of underlying chemical and physical principles, may turn out not to be true. But the molecular and genetic revolutions churning through medicine are illustrating that reductionism is going to have an enormous influence on disease classification. That is not a bad thing, but it is something that is going to take a lot to get used to.
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained
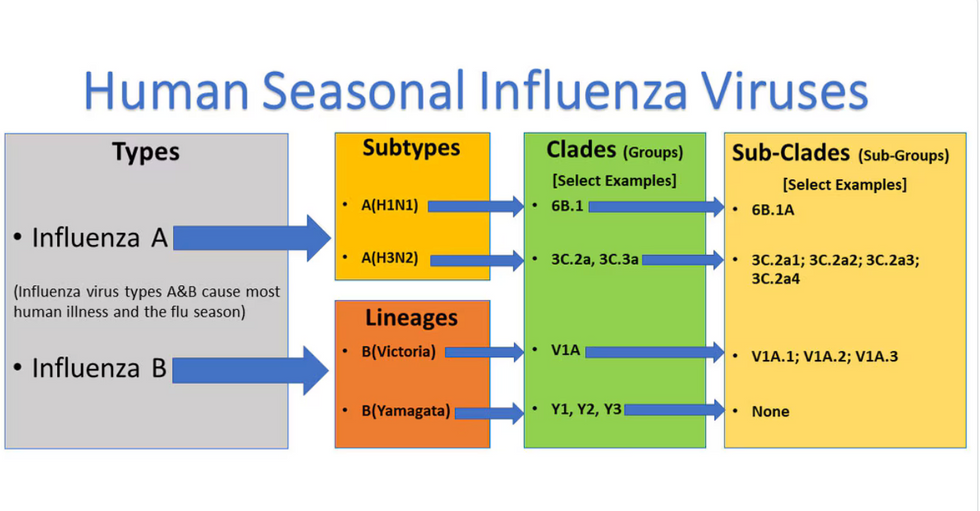
Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

