The Only Hydroxychloroquine Story You Need to Read
Dr. Adalja is focused on emerging infectious disease, pandemic preparedness, and biosecurity. He has served on US government panels tasked with developing guidelines for the treatment of plague, botulism, and anthrax in mass casualty settings and the system of care for infectious disease emergencies, and as an external advisor to the New York City Health and Hospital Emergency Management Highly Infectious Disease training program, as well as on a FEMA working group on nuclear disaster recovery. Dr. Adalja is an Associate Editor of the journal Health Security. He was a coeditor of the volume Global Catastrophic Biological Risks, a contributing author for the Handbook of Bioterrorism and Disaster Medicine, the Emergency Medicine CorePendium, Clinical Microbiology Made Ridiculously Simple, UpToDate's section on biological terrorism, and a NATO volume on bioterrorism. He has also published in such journals as the New England Journal of Medicine, the Journal of Infectious Diseases, Clinical Infectious Diseases, Emerging Infectious Diseases, and the Annals of Emergency Medicine. He is a board-certified physician in internal medicine, emergency medicine, infectious diseases, and critical care medicine. Follow him on Twitter: @AmeshAA
Hydroxychloroquine has been shown not to provide benefit for COVID-19 patients in multiple randomized controlled trials, despite continuous misinformation asserting its alleged effectiveness.
In the early days of a pandemic caused by a virus with no existing treatments, many different compounds are often considered and tried in an attempt to help patients.
It all relates back to a profound question: How do we know what we know?
Many of these treatments fall by the wayside as evidence accumulates regarding actual efficacy. At that point, other treatments become standard of care once their benefit is proven in rigorously designed trials.
However, about seven months into the pandemic, we're still seeing political resurrection of a treatment that has been systematically studied and demonstrated in well-designed randomized controlled trials to not have benefit.
The hydroxychloroquine (and by extension chloroquine) story is a complicated one that was difficult to follow even before it became infused with politics. It is a simple fact that these drugs, long approved by the Food and Drug Administration (FDA), work in Petri dishes against various viruses including coronaviruses. This set of facts provided biological plausibility to support formally studying their use in the clinical treatment and prevention of COVID-19. As evidence from these studies accumulates, it is a cognitive requirement to integrate that knowledge and not to evade it. This also means evaluating the rigor of the studies.
In recent days we have seen groups yet again promoting the use of hydroxychloroquine in, what is to me, a baffling disregard of the multiple recent studies that have shown no benefit. Indeed, though FDA-approved for other indications like autoimmune conditions and preventing malaria, the emergency use authorization for COVID-19 has been rescinded (which means the government cannot stockpile it). Still, however, many patients continue to ask for the drug, compelled by political commentary, viral videos, and anecdotal data. Yet most doctors (like myself) are refusing to write the prescriptions outside of a clinical trial – a position endorsed by professional medical organizations such as the American College of Physicians and the Infectious Diseases Society of America. Why this disconnect?
It all relates back to a profound question: How do we know what we know? In science, we use the scientific method – the process of observing reality, coming up with a hypothesis about what might be true, and testing that hypothesis as thoroughly as possible until we discover the objective truth.
The confusion we're seeing now stems from an inability to distinguish between anecdotes reported by physicians (observational data) and an actual evidence base. This is understandable among the general public but when done by a healthcare professional, it reveals a disdain for reason, logic, and the scientific method.
The Difference Between Observational Data and Randomized Controlled Trials
The power of informal observation is crucial. It is part of the scientific method but primarily as a basis for generating hypotheses that we can test. How do we conduct medical tests? The gold standard is the double-blind, randomized, placebo-controlled trial. This means that neither the researchers nor the volunteers know who is getting a drug and who is getting a sugar pill. Then both groups of the trial, called arms, can be compared to determine whether the people who got the drug fared better. This study design prevents biases and the placebo effect from confounding the data and undermining the veracity of the results.
For example, a seemingly beneficial effect might be seen in an observational study with no blinding and no control group. In such a case, all patients are openly given the drug and their doctors observe how they do. A prime example is the 36-patient single-arm study from France that generated a tremendous amount of interest after President Trump tweeted about it. But this kind of a study by its nature cannot answer the critical question: Was the positive effect because of hydroxychloroquine or just the natural course of the illness? In other words, would someone have recovered in a similar fashion regardless of the drug? What is the role of the placebo effect?
These are reasons why it is crucial to give a placebo to a control group that is as similar in every respect as possible to those receiving the intervention. Then we attempt to find out by comparing the two groups: What is the side effect profile of the drug? Are the groups large enough to detect a relatively rare safety concern? How long were the patients followed for? Was something else responsible for making the patients get better, such as the use of steroids (as likely was the case in the Henry Ford study)?
Looking at the two major hydroxychloroquine trials, it is apparent that, when studied using the best tools of clinical trials, no benefit is likely to occur.
All of these considerations amount to just a fraction of the questions that can be answered more definitively in a well-designed large randomized controlled trial than in observational studies. Indeed, an observational study from New York failed to show any benefit in hospitalized patients, showing how unclear and disparate the results can be with these types of studies. A New York retrospective study (which examined patient outcomes after they were already treated) had similar results and included the use of azithromycin.
When evaluating a study, it is also important to note whether conflicts of interest exist, as well as the quality of the peer review and the data itself. In the case of the French study, for example, the paper was published in a journal in which one of the authors was editor-in-chief, and it was accepted for publication after 24 hours. Patients who fared poorly on hydroxychloroquine were also left out of the study altogether, skewing the results.
What Randomized Controlled Trials Have Shown
Looking at the two major hydroxychloroquine trials, it is apparent that, when studied using the best tools of clinical trials, no benefit is likely to occur. The most important of these studies to announce results was part of the Recovery trial, which was designed to test multiple interventions in the treatment of COVID-19. This trial, which has yet to be formally published, was a randomized controlled trial that involved over 1500 hospitalized patients being administered hydroxychloroquine compared to over 3000 who did not receive the medication. Clinical testing requires large numbers of patients to have the power to demonstrate statistical significance -- the threshold at which any apparent benefit is more than you would expect by random chance alone.
In this study, hydroxychloroquine provided no mortality benefit or even a benefit in hospital length of stay. In fact, the opposite occurred. Hydroxychloroquine patients were more likely to stay in the hospital longer and were more likely to require mechanical ventilation. Additionally, smaller randomized trials conducted in China have not shown benefit either.
Another major study involved the use of hydroxychloroquine to prevent illness in people who were exposed to COVID-19. These results, published in The New England Journal of Medicine, included over 800 patients who were studied in a randomized double-blind controlled trial and also failed to show any benefit.
But what about adding the antibiotic azithromycin in conjunction with hydroxychloroquine? A three-arm randomized controlled study involving over 500 patients hospitalized with mild to moderate COVID-19 was conducted. Its results, also published in The New England Journal of Medicine, failed to show any benefit – with or without azithromycin – and demonstrated evidence of harm. Those who received these treatments had elevations of their liver function tests and heart rhythm abnormalities. These findings hold despite the retraction of an observational study showing similar results.
Additionally, when used in combination with remdesivir – an experimental antiviral – hydroxychloroquine has been shown to be associated with worse outcomes and more side effects.
But what about in mildly ill patients not requiring hospitalization? There was no benefit found in a randomized double-blind placebo-controlled trial of 400 patients, the majority of whom were given the drug within one day of symptoms.
Some randomized controlled studies have yet to report their findings on hydroxychloroquine in non-hospitalized patients, with the use of zinc (which has some evidence in the treatment of the common cold, another ailment that can be caused by coronaviruses). And studies have yet to come out regarding whether hydroxychloroquine can prevent people from getting sick before they are even exposed. But the preponderance of the evidence from studies designed specifically to find benefit for treating COVID-19 does not support its use outside of a research setting.
Today – even with some studies (including those with zinc) still ongoing – if a patient asked me to prescribe them hydroxychloroquine for any severity or stage of illness, with or without zinc, with or without azithromycin, I would refrain. I would explain that, based on the evidence from clinical trials that has been amassed, there is no reason to believe that it will alter the course of illness for the better.
Failing to recognize the reality of the situation runs the risk of crowding out other more promising treatments and creating animosity where none should exist.
What has been occurring is a continual shifting of goalposts with each negative hydroxychloroquine study. Those in favor of the drug protest that a trial did not include azithromycin or zinc or wasn't given at the right time to the right patients. While there may be biological plausibility to treating illness early or combining treatments with zinc, it can only be definitively shown in a randomized, controlled prospective study.
The bottom line: A study that only looks at past outcomes in one group of patients – even when well conducted – is at most hypothesis generating and cannot be used as the sole basis for a new treatment paradigm.
Some may argue that there is no time to wait for definitive studies, but no treatment is benign. The risk/benefit ratio is not the same for every possible use of the drug. For example, hydroxychloroquine has a long record of use in rheumatoid arthritis and systemic lupus (whose patients are facing shortages because of COVID-19 related demand). But the risk of side effects for many of these patients is worth taking because of the substantial benefit the drug provides in treating those conditions.
In COVID-19, however, the disease apparently causes cardiac abnormalities in a great deal of many mild cases, a situation that should prompt caution when using any drugs that have known effects on the cardiac system -- drugs like hydroxychloroquine and azithromycin.
My Own Experience
It is not the case that every physician was biased against this drug from the start. Indeed, most of us wanted it to be shown to be beneficial, as it was a generic drug that was widely available and very familiar. In fact, early in the pandemic I prescribed it to hospitalized patients on two occasions per a hospital protocol. However, it is impossible for me as a sole clinician to know whether it worked, was neutral, or was harmful. In recent days, however, I have found the hydroxychloroquine talk to have polluted the atmosphere. One recent patient was initially refusing remdesivir, a drug proven in large randomized trials to have effectiveness, because he had confused it with hydroxychloroquine.
Moving On to Other COVID Treatments: What a Treatment Should Do
The story of hydroxychloroquine illustrates a fruitless search for what we are actually looking for in a COVID-19 treatment. In short, we are looking for a medication that can decrease symptoms, decrease complications, hasten recovery, decrease hospitalizations, decrease contagiousness, decrease deaths, and prevent infection. While it is unlikely to find a single antiviral that can accomplish all of these, fulfilling even just one is important.
For example, remdesivir hastens recovery and dexamethasone decreases mortality. Definitive results of the use of convalescent plasma and immunomodulating drugs such as siltuxamab, baricitinib, and anakinra (for use in the cytokine storms characteristic of severe disease) are still pending, as are the trials with monoclonal antibodies.
While it was crucial that the medical and scientific community definitively answer the questions surrounding the use of chloroquine and hydroxychloroquine in the treatment of COVID-19, it is time to face the facts and accept that its use for the treatment of this disease is not likely to be beneficial. Failing to recognize the reality of the situation runs the risk of crowding out other more promising treatments and creating animosity where none should exist.
Dr. Adalja is focused on emerging infectious disease, pandemic preparedness, and biosecurity. He has served on US government panels tasked with developing guidelines for the treatment of plague, botulism, and anthrax in mass casualty settings and the system of care for infectious disease emergencies, and as an external advisor to the New York City Health and Hospital Emergency Management Highly Infectious Disease training program, as well as on a FEMA working group on nuclear disaster recovery. Dr. Adalja is an Associate Editor of the journal Health Security. He was a coeditor of the volume Global Catastrophic Biological Risks, a contributing author for the Handbook of Bioterrorism and Disaster Medicine, the Emergency Medicine CorePendium, Clinical Microbiology Made Ridiculously Simple, UpToDate's section on biological terrorism, and a NATO volume on bioterrorism. He has also published in such journals as the New England Journal of Medicine, the Journal of Infectious Diseases, Clinical Infectious Diseases, Emerging Infectious Diseases, and the Annals of Emergency Medicine. He is a board-certified physician in internal medicine, emergency medicine, infectious diseases, and critical care medicine. Follow him on Twitter: @AmeshAA
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
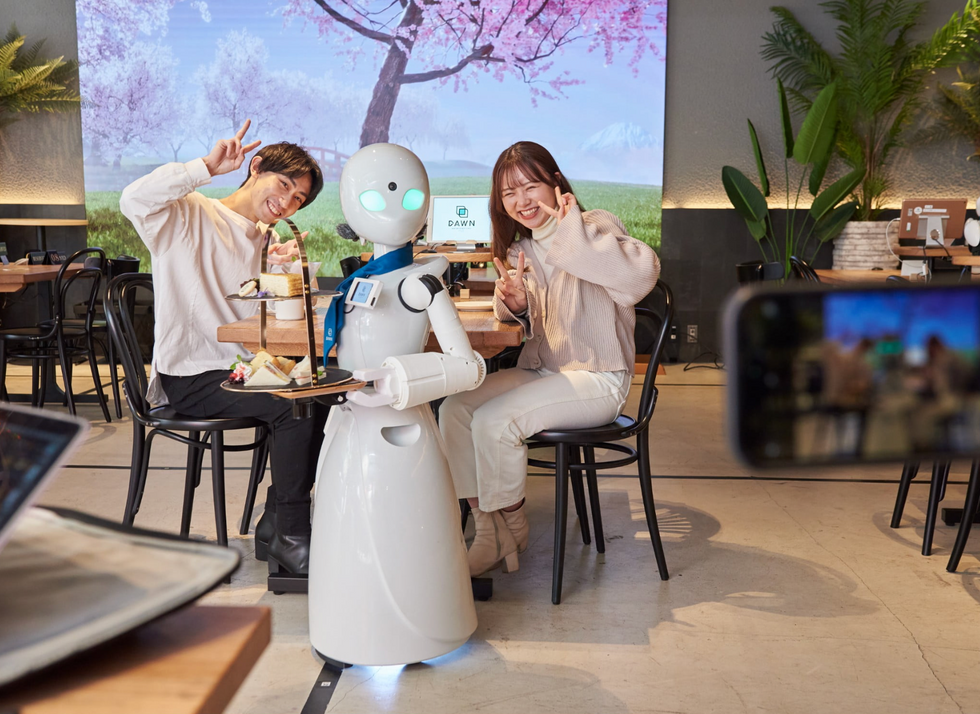
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
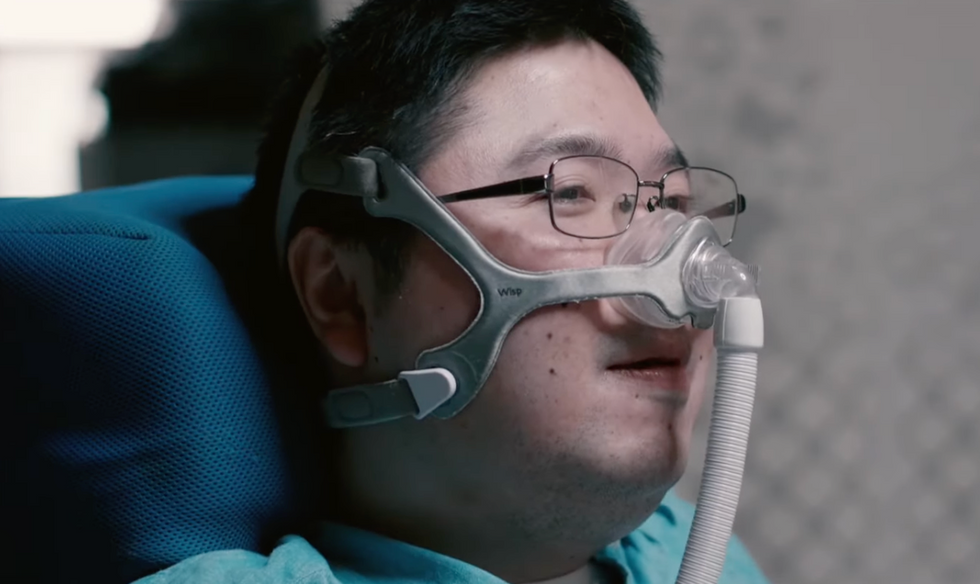
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

