Want to Motivate Vaccinations? Message Optimism, Not Doom

There is a lot to be optimistic about regarding the new safe and highly effective vaccines, which are moving society closer toward the goal of close human contact once again.
After COVID-19 was declared a worldwide pandemic by the World Health Organization on March 11, 2020, life as we knew it altered dramatically and millions went into lockdown. Since then, most of the world has had to contend with masks, distancing, ventilation and cycles of lockdowns as surges flare up. Deaths from COVID-19 infection, along with economic and mental health effects from the shutdowns, have been devastating. The need for an ultimate solution -- safe and effective vaccines -- has been paramount.
On November 9, 2020 (just 8 months after the pandemic announcement), the press release for the first effective COVID-19 vaccine from Pfizer/BioNTech was issued, followed by positive announcements regarding the safety and efficacy of five other vaccines from Moderna, University of Oxford/AztraZeneca, Novavax, Johnson and Johnson and Sputnik V. The Moderna and Pfizer vaccines have earned emergency use authorization through the FDA in the United States and are being distributed. We -- after many long months -- are seeing control of the devastating COVID-19 pandemic glimmering into sight.
To be clear, these vaccine candidates for COVID-19, both authorized and not yet authorized, are highly effective and safe. In fact, across all trials and sites, all six vaccines were 100% effective in preventing hospitalizations and death from COVID-19.
All Vaccines' Phase 3 Clinical Data
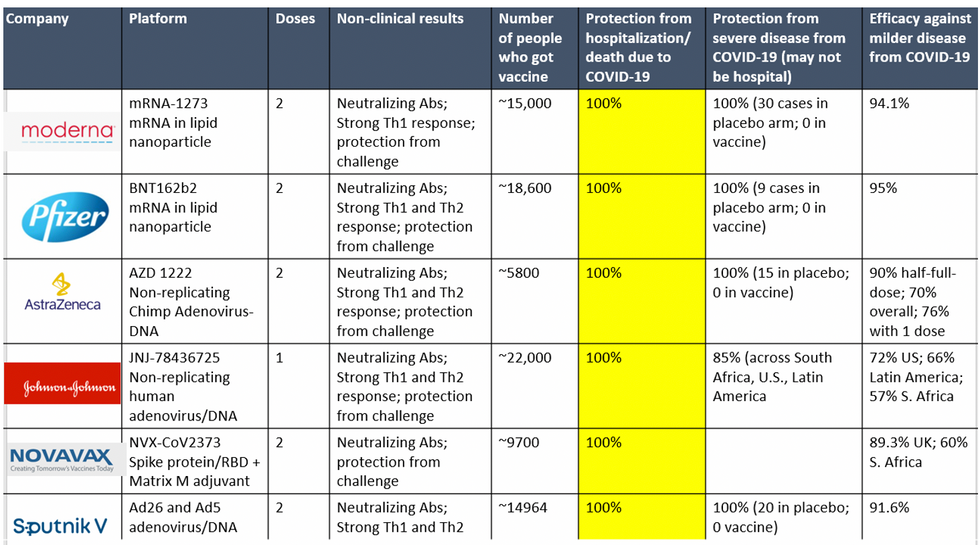
Complete protection against hospitalization and death from COVID-19 exhibited by all vaccines with phase 3 clinical trial data.
This astounding level of protection from SARS-CoV-2 from all vaccine candidates across multiple regions is likely due to robust T cell response from vaccination and will "defang" the virus from the concerns that led to COVID-19 restrictions initially: the ability of the virus to cause severe illness. This is a time of hope and optimism. After the devastating third surge of COVID-19 infections and deaths over the winter, we finally have an opportunity to stem the crisis – if only people readily accept the vaccines.
Amidst these incredible scientific advancements, however, public health officials and politicians have been pushing downright discouraging messaging. The ubiquitous talk of ongoing masks and distancing restrictions without any clear end in sight threatens to dampen uptake of the vaccines. It's imperative that we break down each concern and see if we can revitalize our public health messaging accordingly.
The first concern: we currently do not know if the vaccines block asymptomatic infection as well as symptomatic disease, since none of the phase 3 vaccine trials were set up to answer this question. However, there is biological plausibility that the antibodies and T-cell responses blocking symptomatic disease will also block asymptomatic infection in the nasal passages. IgG immunoglobulins (generated and measured by the vaccine trials) enter the nasal mucosa and systemic vaccinations generate IgA antibodies at mucosal surfaces. Monoclonal antibodies given to outpatients with COVID-19 hasten viral clearance from the airways.
Although it is prudent for those who are vaccinated to wear masks around the unvaccinated in case a slight risk of transmission remains, two fully vaccinated people can comfortably abandon masking around each other.
Moreover, data from the AztraZeneca trial (including in the phase 3 trial final results manuscript), where weekly self-swabbing was done by participants, and data from the Moderna trial, where a nasal swab was performed prior to the second dose, both showed risk reductions in asymptomatic infection with even a single dose. Finally, real-world data from a large Pfizer-based vaccine campaign in Israel shows a 50% reduction in infections (asymptomatic or symptomatic) after just the first dose.
Therefore, the likelihood of these vaccines blocking asymptomatic carriage, as well as symptomatic disease, is high. Although it is prudent for those who are vaccinated to wear masks around the unvaccinated in case a slight risk of transmission remains, two fully vaccinated people can comfortably abandon masking around each other. Moreover, as the percentage of vaccinated people increases, it will be increasingly untenable to impose restrictions on this group. Once herd immunity is reached, these restrictions can and should be abandoned altogether.
The second concern translating to "doom and gloom" messaging lately is around the identification of troubling new variants due to enhanced surveillance via viral sequencing. Four major variants circulating at this point (with others described in the past) are the B.1.1.7 variant ("UK variant"), B.1.351 ("South Africa variant), P.1. ("Brazil variant"), and the L452R variant identified in California. Although the UK variant is likely to be more transmissible, as is the South Africa variant, we have no reason to believe that masks, distancing and ventilation are ineffective against these variants.
Moreover, neutralizing antibody titers with the Pfizer and Moderna vaccines do not seem to be significantly reduced against the variants. Finally, although the Novavax 2-dose and Johnson and Johnson (J&J) 1-dose vaccines had lower rates of efficacy against moderate COVID-19 disease in South Africa, their efficacy against severe disease was impressively high. In fact J&J's vaccine still prevented 100% of hospitalizations and death from COVID-19. When combining both hospitalizations/deaths and severe symptoms managed at home, the J&J 1-dose vaccine was 85% protective across all three sites of the trial: the U.S., Latin America (including Brazil), and South Africa.
In South Africa, nearly all cases of COVID-19 (95%) were due to infection with the B.1.351 SARS-CoV-2 variant. Finally, since herd immunity does not rely on maximal immune responses among all individuals in a society, the Moderna/Pfizer/J&J vaccines are all likely to achieve that goal against variants. And thankfully, all of these vaccines can be easily modified to boost specifically against a new variant if needed (indeed, Moderna and Pfizer are already working on boosters against the prominent variants).
The third concern of some public health officials is that people will abandon all restrictions once vaccinated unless overly cautious messages are drilled into them. Indeed, the false idea that if you "give people an inch, they will take a mile" has been misinforming our messaging about mitigation since the beginning of the pandemic. For example, the very phrase "stay at home" with all of its non-applicability for essential workers and single individuals is stigmatizing and unrealistic for many. Instead, the message should have focused on how people can additively reduce their risks under different circumstances.
The public will be more inclined to trust health officials if those officials communicate with nuanced messages backed up by evidence, rather than with broad brushstrokes that shame. Therefore, we should be saying that "vaccinated people can be together with other vaccinated individuals without restrictions but must protect the unvaccinated with masks and distancing." And we can say "unvaccinated individuals should adhere to all current restrictions until vaccinated" without fear of misunderstandings. Indeed, this kind of layered advice has been communicated to people living with HIV and those without HIV for a long time (if you have HIV but partner does not, take these precautions; if both have HIV, you can do this, etc.).
Our heady progress in vaccine development, along with the incredible efficacy results of all of them, is unprecedented. However, we are at risk of undermining such progress if people balk at the vaccine because they don't believe it will make enough of a difference. One of the most critical messages we can deliver right now is that these vaccines will eventually free us from the restrictions of this pandemic. Let's use tiered messaging and clear communication to boost vaccine optimism and uptake, and get us to the goal of close human contact once again.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
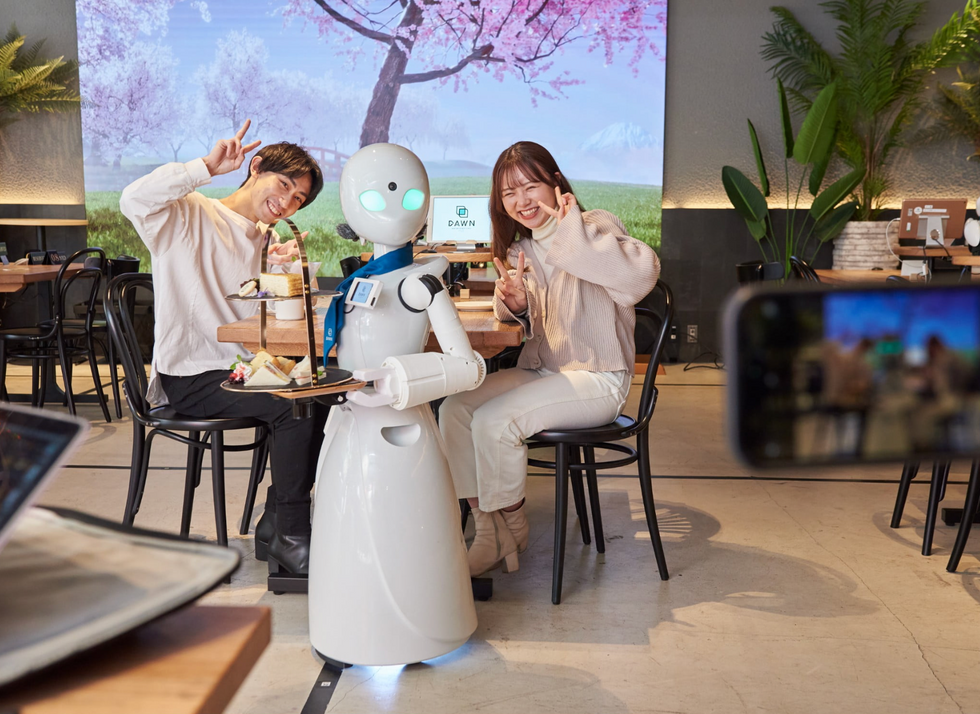
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
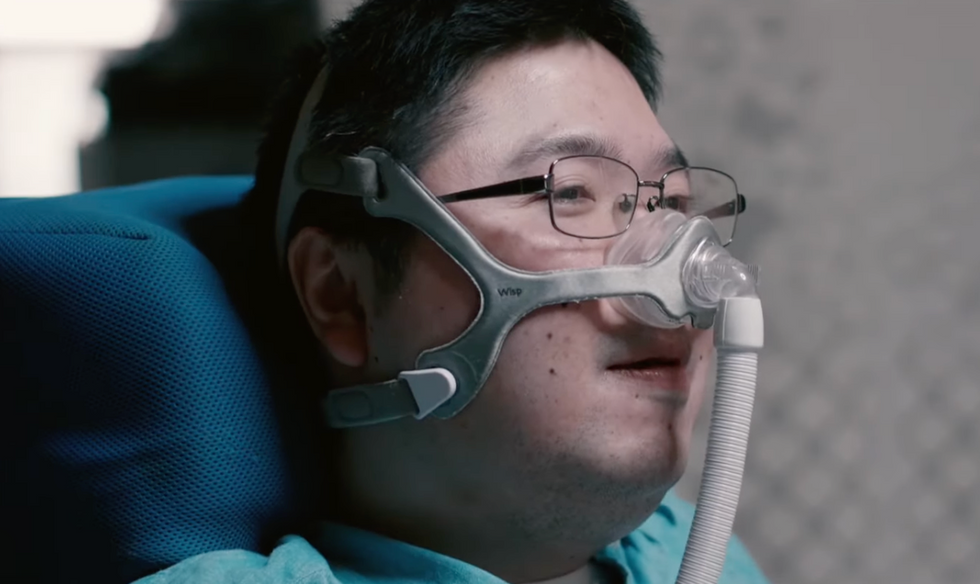
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

