3 Futuristic Biotech Programs the U.S. Government Is Funding Right Now
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

Biomedical engineer Kevin Zhao has a sensor in his arm and his chest that monitors his oxygen level in those tissues in real time.
Last month, at a conference celebrating DARPA, the research arm of the Defense Department, FBI Special Agent Edward You declared, "The 21st century will be the revolution of the life sciences."
Biomedical engineer Kevin Zhao has a sensor in his arm and chest that monitors his oxygen level in real time.
Indeed, four years ago, the agency dedicated a new office solely to advancing biotechnology. Its primary goal is to combat bioterrorism, protect U.S. forces, and promote warfighter readiness. But its research could also carry over to improve health care for the general public.
With an annual budget of about $3 billion, DARPA's employees oversee about 250 research and development programs, working with contractors from corporations, universities, and government labs to bring new technologies to life.
Check out these three current programs:
1) IMPLANTABLE SENSORS TO MEASURE OXYGEN, LACTATE, AND GLUCOSE LEVELS IN REAL TIME
Biomedical engineer Kevin Zhao has a sensor in his arm and his chest that monitors his oxygen level in those tissues in real time. With funding from DARPA for the program "In Vivo Nanoplatforms," he developed soft, flexible hydrogels that are injected just beneath the skin to perform the monitoring and that sync to a smartphone app to give the user immediate health insights.
A first-in-man trial for the glucose sensor is now underway in Europe for monitoring diabetics, according to Zhao. Volunteers eat sugary food to spike their glucose levels and prompt the monitor to register the changes.
"If this pans out, with approval from FDA, then consumers could get the sensors implanted in their core to measure their levels of glucose, oxygen, and lactate," Zhao said.
Lactate, especially, interests DARPA because it's a first responder molecule to the onset of trauma, sepsis, and potentially infection.
"The sensor could potentially detect rise of these [body chemistry numbers] and alert the user to prevent onset of dangerous illness."
2) NEAR INSTANTANEOUS VACCINE PROTECTION DURING A PANDEMIC
Traditional vaccines can take months or years to develop, then weeks to become effective once you get it. But when an unknown virus emerges, there's no time to waste.
This program, called P3, envisions a much more ambitious approach to stop a pandemic in its tracks.
"We want to confer near instantaneous protection by doing it a different way – enlist the body as a bioreactor to produce therapeutics," said Col. Matthew Hepburn, the program manager.
So how would it work?
To fight a pandemic, we will need 20,000 doses of a vaccine in 60 days.
If you have antibodies against a certain infection, you'll be protected against that infection. This idea is to discover the genetic code for the antibody to a specific pathogen, manufacture those pieces of DNA and RNA, and then inject the code into a person's arm so the muscle cells will begin producing the required antibodies.
"The amazing thing is that it actually works, at least in animal models," said Hepburn. "The mouse muscles made enough protective antibodies so that the mice were protected."
The next step is to test the approach in humans, which the program will do over the next two years.
But the hard part is actually not discovering the genetic code for highly potent antibodies, according to Hepburn. In fact, researchers already have been able to do so in two to four weeks' time.
"The hard part is once I have an antibody, a large pharma company will say in 2 years, I can make 100-200 doses. Give us 4 years to get to 20,000 doses. That's not good enough," Hepburn said.
To fight a pandemic, we will need 20,000 doses of a vaccine in 60 days.
"We have to fundamentally change the idea that it takes a billion dollars and ten years to make a drug," he concluded. "We're going to do something radically different."
3) RAPID DIAGNOSING OF PATHOGEN EXPOSURE THROUGH EPIGENETICS
Imagine that you come down with a mysterious illness. It could be caused by a virus, bacteria, or in the most extreme catastrophe, a biological agent from a weapon of mass destruction.
What if a portable device existed that could identify--within 30 minutes—which pathogen you have been exposed to and when? It would be pretty remarkable for soldiers in the field, but also for civilians seeking medical treatment.
This is the lofty ambition of a DARPA program called Epigenetic Characterization and Observation, or ECHO.
Its success depends on a biological phenomenon known as the epigenome. While your DNA is relatively immutable, your environment can modify how your DNA is expressed, leaving marks of exposure that register within seconds to minutes; these marks can persist for decades. It's thanks to the epigenome that identical twins – who share identical DNA – can differ in health, temperament, and appearance.

These three mice are genetically identical. Epigenetic differences, however, result in vastly different observed characteristics.
Reading your epigenetic marks could theoretically reveal a time-stamped history of your body's environmental exposures.
Researchers in the ECHO program plan to create a database of signatures for exposure events, so that their envisioned device will be able to quickly scan someone's epigenome and refer to the database to sort out a diagnosis.
"One difficult part is to put a timestamp on this result, in addition to the sign of which exposure it was -- to tell us when this exposure happened," says Thomas Thomou, a contract scientist who is providing technical assistance to the ECHO program manager.
Other questions that remain up in the air for now: Do all humans have the same epigenetic response to the same exposure events? Is it possible to distinguish viral from bacterial exposures? Does dose and duration of exposure affect the signature of epigenome modification?
The program will kick off in January 2019 and is planned to last four years, as long as certain milestones of development are reached along the way. The desired prototype would be a simple device that any untrained person could operate by taking a swab or a fingerprick.
"In an outbreak," says Dr. Thomou, "it will help everyone on the ground immediately to have a rapidly deployable machine that will give you very quick answers to issues that could have far-reaching ramifications for public health safety."
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Dr. May Edward Chinn, Kizzmekia Corbett, PhD., and Alice Ball, among others, have been behind some of the most important scientific work of the last century.
If you look back on the last century of scientific achievements, you might notice that most of the scientists we celebrate are overwhelmingly white, while scientists of color take a backseat. Since the Nobel Prize was introduced in 1901, for example, no black scientists have landed this prestigious award.
The work of black women scientists has gone unrecognized in particular. Their work uncredited and often stolen, black women have nevertheless contributed to some of the most important advancements of the last 100 years, from the polio vaccine to GPS.
Here are five black women who have changed science forever.
Dr. May Edward Chinn
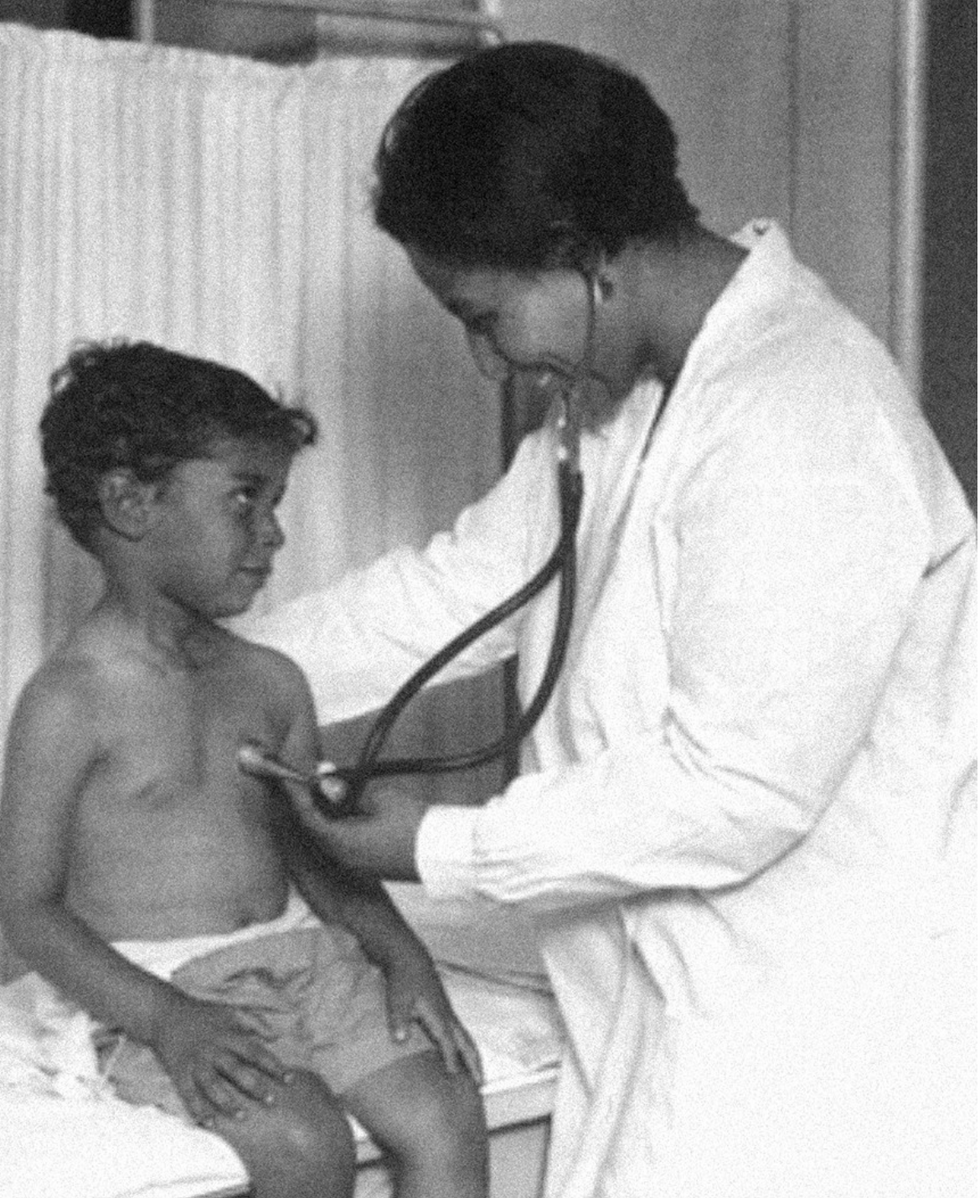
Dr. May Edward Chinn practicing medicine in Harlem
George B. Davis, PhD.
Chinn was born to poor parents in New York City just before the start of the 20th century. Although she showed great promise as a pianist, playing with the legendary musician Paul Robeson throughout the 1920s, she decided to study medicine instead. Chinn, like other black doctors of the time, were barred from studying or practicing in New York hospitals. So Chinn formed a private practice and made house calls, sometimes operating in patients’ living rooms, using an ironing board as a makeshift operating table.
Chinn worked among the city’s poor, and in doing this, started to notice her patients had late-stage cancers that often had gone undetected or untreated for years. To learn more about cancer and its prevention, Chinn begged information off white doctors who were willing to share with her, and even accompanied her patients to other clinic appointments in the city, claiming to be the family physician. Chinn took this information and integrated it into her own practice, creating guidelines for early cancer detection that were revolutionary at the time—for instance, checking patient health histories, checking family histories, performing routine pap smears, and screening patients for cancer even before they showed symptoms. For years, Chinn was the only black female doctor working in Harlem, and she continued to work closely with the poor and advocate for early cancer screenings until she retired at age 81.
Alice Ball

Pictorial Press Ltd/Alamy
Alice Ball was a chemist best known for her groundbreaking work on the development of the “Ball Method,” the first successful treatment for those suffering from leprosy during the early 20th century.
In 1916, while she was an undergraduate student at the University of Hawaii, Ball studied the effects of Chaulmoogra oil in treating leprosy. This oil was a well-established therapy in Asian countries, but it had such a foul taste and led to such unpleasant side effects that many patients refused to take it.
So Ball developed a method to isolate and extract the active compounds from Chaulmoogra oil to create an injectable medicine. This marked a significant breakthrough in leprosy treatment and became the standard of care for several decades afterward.
Unfortunately, Ball died before she could publish her results, and credit for this discovery was given to another scientist. One of her colleagues, however, was able to properly credit her in a publication in 1922.
Henrietta Lacks

onathan Newton/The Washington Post/Getty
The person who arguably contributed the most to scientific research in the last century, surprisingly, wasn’t even a scientist. Henrietta Lacks was a tobacco farmer and mother of five children who lived in Maryland during the 1940s. In 1951, Lacks visited Johns Hopkins Hospital where doctors found a cancerous tumor on her cervix. Before treating the tumor, the doctor who examined Lacks clipped two small samples of tissue from Lacks’ cervix without her knowledge or consent—something unthinkable today thanks to informed consent practices, but commonplace back then.
As Lacks underwent treatment for her cancer, her tissue samples made their way to the desk of George Otto Gey, a cancer researcher at Johns Hopkins. He noticed that unlike the other cell cultures that came into his lab, Lacks’ cells grew and multiplied instead of dying out. Lacks’ cells were “immortal,” meaning that because of a genetic defect, they were able to reproduce indefinitely as long as certain conditions were kept stable inside the lab.
Gey started shipping Lacks’ cells to other researchers across the globe, and scientists were thrilled to have an unlimited amount of sturdy human cells with which to experiment. Long after Lacks died of cervical cancer in 1951, her cells continued to multiply and scientists continued to use them to develop cancer treatments, to learn more about HIV/AIDS, to pioneer fertility treatments like in vitro fertilization, and to develop the polio vaccine. To this day, Lacks’ cells have saved an estimated 10 million lives, and her family is beginning to get the compensation and recognition that Henrietta deserved.
Dr. Gladys West

Andre West
Gladys West was a mathematician who helped invent something nearly everyone uses today. West started her career in the 1950s at the Naval Surface Warfare Center Dahlgren Division in Virginia, and took data from satellites to create a mathematical model of the Earth’s shape and gravitational field. This important work would lay the groundwork for the technology that would later become the Global Positioning System, or GPS. West’s work was not widely recognized until she was honored by the US Air Force in 2018.
Dr. Kizzmekia "Kizzy" Corbett

TIME Magazine
At just 35 years old, immunologist Kizzmekia “Kizzy” Corbett has already made history. A viral immunologist by training, Corbett studied coronaviruses at the National Institutes of Health (NIH) and researched possible vaccines for coronaviruses such as SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East Respiratory Syndrome).
At the start of the COVID pandemic, Corbett and her team at the NIH partnered with pharmaceutical giant Moderna to develop an mRNA-based vaccine against the virus. Corbett’s previous work with mRNA and coronaviruses was vital in developing the vaccine, which became one of the first to be authorized for emergency use in the United States. The vaccine, along with others, is responsible for saving an estimated 14 million lives.On today’s episode of Making Sense of Science, I’m honored to be joined by Dr. Paul Song, a physician, oncologist, progressive activist and biotech chief medical officer. Through his company, NKGen Biotech, Dr. Song is leveraging the power of patients’ own immune systems by supercharging the body’s natural killer cells to make new treatments for Alzheimer’s and cancer.
Whereas other treatments for Alzheimer’s focus directly on reducing the build-up of proteins in the brain such as amyloid and tau in patients will mild cognitive impairment, NKGen is seeking to help patients that much of the rest of the medical community has written off as hopeless cases, those with late stage Alzheimer’s. And in small studies, NKGen has shown remarkable results, even improvement in the symptoms of people with these very progressed forms of Alzheimer’s, above and beyond slowing down the disease.
In the realm of cancer, Dr. Song is similarly setting his sights on another group of patients for whom treatment options are few and far between: people with solid tumors. Whereas some gradual progress has been made in treating blood cancers such as certain leukemias in past few decades, solid tumors have been even more of a challenge. But Dr. Song’s approach of using natural killer cells to treat solid tumors is promising. You may have heard of CAR-T, which uses genetic engineering to introduce cells into the body that have a particular function to help treat a disease. NKGen focuses on other means to enhance the 40 plus receptors of natural killer cells, making them more receptive and sensitive to picking out cancer cells.
Paul Y. Song, MD is currently CEO and Vice Chairman of NKGen Biotech. Dr. Song’s last clinical role was Asst. Professor at the Samuel Oschin Cancer Center at Cedars Sinai Medical Center.
Dr. Song served as the very first visiting fellow on healthcare policy in the California Department of Insurance in 2013. He is currently on the advisory board of the Pritzker School of Molecular Engineering at the University of Chicago and a board member of Mercy Corps, The Center for Health and Democracy, and Gideon’s Promise.
Dr. Song graduated with honors from the University of Chicago and received his MD from George Washington University. He completed his residency in radiation oncology at the University of Chicago where he served as Chief Resident and did a brachytherapy fellowship at the Institute Gustave Roussy in Villejuif, France. He was also awarded an ASTRO research fellowship in 1995 for his research in radiation inducible gene therapy.
With Dr. Song’s leadership, NKGen Biotech’s work on natural killer cells represents cutting-edge science leading to key findings and important pieces of the puzzle for treating two of humanity’s most intractable diseases.
Show links
- Paul Song LinkedIn
- NKGen Biotech on Twitter - @NKGenBiotech
- NKGen Website: https://nkgenbiotech.com/
- NKGen appoints Paul Song
- Patient Story: https://pix11.com/news/local-news/long-island/promising-new-treatment-for-advanced-alzheimers-patients/
- FDA Clearance: https://nkgenbiotech.com/nkgen-biotech-receives-ind-clearance-from-fda-for-snk02-allogeneic-natural-killer-cell-therapy-for-solid-tumors/Q3 earnings data: https://www.nasdaq.com/press-release/nkgen-biotech-inc.-reports-third-quarter-2023-financial-results-and-business