A Cure for Sickle Cell Disease Is Coming. Will Patients Accept It?
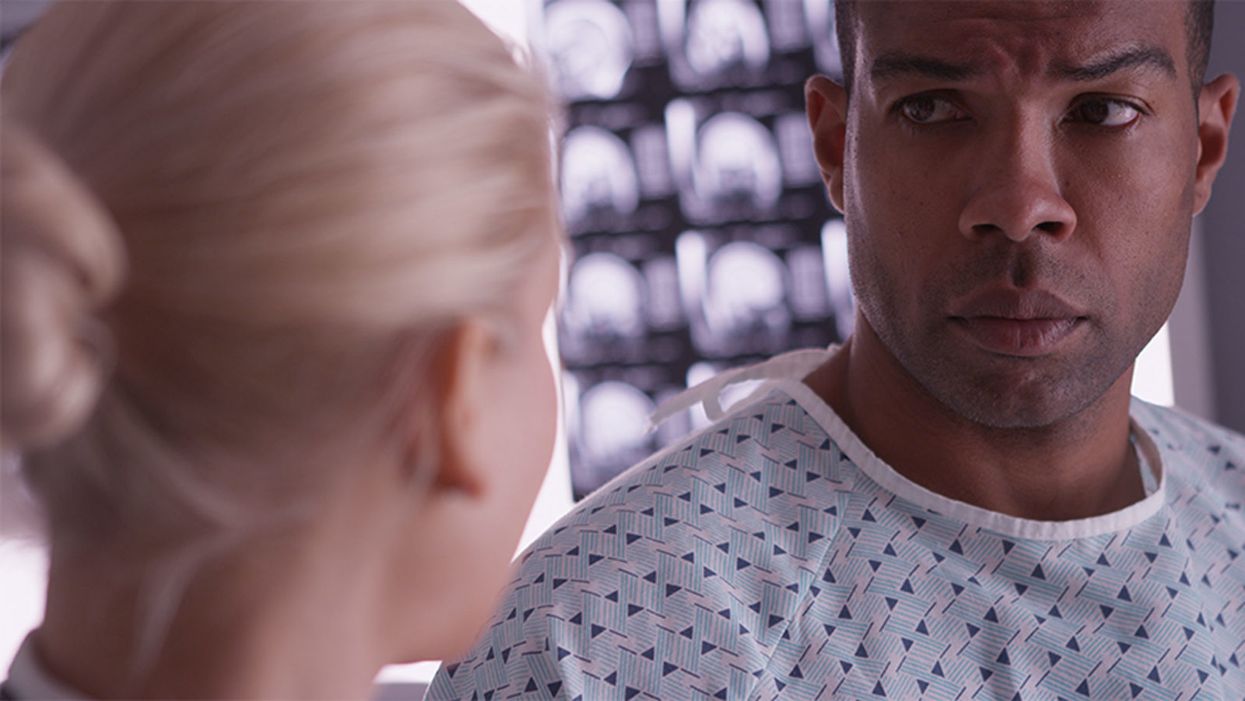
Many patients in the African-American community are skeptical of new experimental treatments, thanks to a history of medical exploitation in the 20th century.
If any malady proves the fragile grace of the human genome, it is sickle cell disease.
If experimental treatments receive regulatory approval, it would be a watershed breakthrough for tens of thousands of Americans.
It occurs because of a single "misspelled" letter of DNA, causing red blood cells to run low on oxygen and transforming the hemoglobin in each cell into a stiff rod. Normally round cells become rigid crescents that hamper the flow of blood throughout the body, like leaves clumping in a drain.
Strokes in toddlers are merely the beginning of the circulatory calamities this disease may inflict. Most sickled cells cannot carry oxygen through the body, causing anemia as well as excruciating chronic pain. Older patients are at risk of kidney failure, heart disease and all the other collateral damage caused by poor circulation. Few live beyond middle age.
The only way to cure it has been through a bone marrow transplant from a donor, which requires not only a closely matching volunteer, but bouts of chemotherapy to allow new stem cells to take root, as well as rounds of immunosuppressive drugs that may last for years.
Recent advances in genomic medicine may soon alter the disease's outlook, although many obstacles remain.
In one treatment under development, patient's skin cells are converted into stem cells, allowing them to be inserted into the bone marrow without the need for a donor. Another treatment known as gene therapy involves replacing the aberrant gene in the patient's body with new genetic material.
Although both remain in clinical trials -- and also require at least chemotherapy -- they have shown promise. Matthew Hsieh, a hematologist and staff scientist with the National Heart Lung and Blood Institute in Maryland, has performed about 10 gene therapy procedures over the past three years as part of a clinical trial. Ongoing tweaks in the procedure have led to the blood in more recent patients showing sickle cell trait -- not a perfect outcome, but one that leaves patients with far fewer symptoms than if they have the full-blown disease.
If one or both treatments receive regulatory approval, it would be a watershed breakthrough for the tens of thousands of Americans who suffer from the disease.
Yet it is entirely possible many patients may decline the cure.
A Painful History
The vast majority of sickle cell sufferers in the U.S. -- well beyond 90 percent -- are African-American, a population with a historically uneasy relationship toward healthcare.
"There is a lot of data on distrust between African-Americans and American medical institutions," says J. Corey Williams, a psychiatrist with the Children's Hospital of Philadelphia who has written extensively on racial disparities in healthcare. "It comes from a long legacy of feeling victimized by medicine."
"What you hear from many patients is 'I am not going to be your guinea pig, and I am not going to be experimented on.'"
As a result, Williams is among several clinicians interviewed for this story who believe a cure for sickle cell disease would be embraced reluctantly.
"What you hear from many patients is 'I am not going to be your guinea pig, and I am not going to be experimented on.' And so the history of African-Americans and research will manifest as we develop gene therapies for [these] patients," says Christopher L. Edwards, a clinical psychologist and researcher with the Maya Angelou Center for Health Equity at the Wake Forest University School of Medicine.
Fear among African-Americans of becoming guinea pigs is well-founded. The first c-sections and fistula repairs occurring in North America were performed on enslaved women -- all without consent and virtually none with anesthesia.
Modern 20th century medicine led to the Tuskegee syphilis experiments conducted by the U.S. Public Health Service. Researchers withheld treatment from some 400 African-American men from the 1930s well into the 1970s to observe how they reacted to the disease -- even though curative antibiotics had been around for decades. Only news reports ended the experiment.
The long-standing distrust of American healthcare in the African-American community is also baked into the care provided to sickle cell patients. Despite affecting one in 365 African-Americans, there is no disease registry to assist clinical trials, according to Mary Hulihan, a blood disorders epidemiologist with the Centers for Disease Control and Prevention. Edwards says many sufferers are suspicious of being monitored.
Meanwhile, only two drugs are available to alleviate the worst symptoms. The first one, hydroxyurea, received FDA approval only in 1998 -- nearly 90 years after the disease was first diagnosed. Moreover, Edwards says that some sufferers shy away from using hydroxyurea because it is also used to treat cancer. It's part of what he calls the "myth and folklore" in the African-American community about sickle cell disease.
Economics plays a role as well in the often-fragmented care such patients receive. According to CDC data, many patients rely extensively on public insurance programs such as Medicaid, whose coverage varies from state to state.
A Tough Transition
Edwards notes that sickle cell sufferers usually receive good care when they're children because of support provided by family members. But that often breaks down in adulthood. According to CDC data, an adult sickle cell patient visits a hospital emergency room three times as often as a child patient.
The consensus is that the path to a medical cure for sickle cell will first need to be smoothed over with a talk cure.
Modupe Idowu, a hematologist with the University of Texas Health system, estimates that there are perhaps a dozen comprehensive care centers for the estimated 100,000 sickle cell patients in the U.S., including the one she operates in Houston. That means a significant proportion of those afflicted are on their own to procure care.
And since many patients are on Medicaid, "a lot of hematologists that train to take care of blood disorders, many are not interested in treating [sickle cell disease] because the reimbursement for providers is not great," Idowu says.
Hsieh acknowledges that many of his patients can be suspicious about the care they are receiving. Frustration with fragmented care is usually the biggest driver, he adds.
Meanwhile, the skepticism that patients have about the treatments they seek is often reciprocated by their caregivers.
"The patients have experiences with medication and know what works at a very young age (for their pain)," Edwards says. Such expertise demonstrated by an African-American patient often leads to them being labeled as narcotics seekers.
The Correct Path
This all begs the question of how to deploy a cure. Idowu, who regularly holds town hall-style meetings with Houston-area patients, often must allay anxieties. For example, the gene therapy approach uses a harmless virus to transport new genetic material into cells. That virus happens to be a benign version of HIV, and convincing patients they won't be infected with HIV is a fraught issue.
The consensus is that the path to a medical cure for sickle cell will first need to be smoothed over with a talk cure.
Idowu tries to hammer home the fact that patients are afforded vastly more protections than in the past. "There are a lot of committees and investigational review boards that keep track of clinical trials; things just don't happen anymore as they did in the past," she says. She also believes it helps if more providers of color communicate to patients.
Hsieh is very straightforward with his patients. He informs them about the HIV vector but assures them no one has ever tested positive for the virus as a result of its use.
Edwards notes that since many patients suffer psychosocial trauma as a result of their chronic pain, there already is some counseling infrastructure in place to help them cope. He believes such resources will have to be stretched further as a cure looms closer.
In the absence of formal mental health services, straight talk may be the best way to overcome wariness.
"If patients have misgivings, we try our best to address them, and let them know at the end of the day it is their decision to make," Hsieh says. "And even the patients who have gone through the gene therapy and it didn't work well -- they're still glad they took the chance."
Gene Transfer Leads to Longer Life and Healthspan
In August, a study provided the first proof-of-principle that genetic material transferred from one species to another can increase both longevity and healthspan in the recipient animal.
The naked mole rat won’t win any beauty contests, but it could possibly win in the talent category. Its superpower: fighting the aging process to live several times longer than other animals its size, in a state of youthful vigor.
It’s believed that naked mole rats experience all the normal processes of wear and tear over their lifespan, but that they’re exceptionally good at repairing the damage from oxygen free radicals and the DNA errors that accumulate over time. Even though they possess genes that make them vulnerable to cancer, they rarely develop the disease, or any other age-related disease, for that matter. Naked mole rats are known to live for over 40 years without any signs of aging, whereas mice live on average about two years and are highly prone to cancer.
Now, these remarkable animals may be able to share their superpower with other species. In August, a study provided what may be the first proof-of-principle that genetic material transferred from one species can increase both longevity and healthspan in a recipient animal.
There are several theories to explain the naked mole rat’s longevity, but the one explored in the study, published in Nature, is based on the abundance of large-molecule high-molecular mass hyaluronic acid (HMM-HA).
A small molecule version of hyaluronic acid is commonly added to skin moisturizers and cosmetics that are marketed as ways to keep skin youthful, but this version, just applied to the skin, won’t have a dramatic anti-aging effect. The naked mole rat has an abundance of the much-larger molecule, HMM-HA, in the chemical-rich solution between cells throughout its body. But does the HMM-HA actually govern the extraordinary longevity and healthspan of the naked mole rat?
To answer this question, Dr. Vera Gorbunova, a professor of biology and oncology at the University of Rochester, and her team created a mouse model containing the naked mole rat gene hyaluronic acid synthase 2, or nmrHas2. It turned out that the mice receiving this gene during their early developmental stage also expressed HMM-HA.
The researchers found that the effects of the HMM-HA molecule in the mice were marked and diverse, exceeding the expectations of the study’s co-authors. High-molecular mass hyaluronic acid was more abundant in kidneys, muscles and other organs of the Has2 mice compared to control mice.
In addition, the altered mice had a much lower incidence of cancer. Seventy percent of the control mice eventually developed cancer, compared to only 57 percent of the altered mice, even after several techniques were used to induce the disease. The biggest difference occurred in the oldest mice, where the cancer incidence for the Has2 mice and the controls was 47 percent and 83 percent, respectively.
With regard to longevity, Has2 males increased their lifespan by more than 16 percent and the females added 9 percent. “Somehow the effect is much more pronounced in male mice, and we don’t have a perfect answer as to why,” says Dr. Gorbunova. Another improvement was in the healthspan of the altered mice: the number of years they spent in a state of relative youth. There’s a frailty index for mice, which includes body weight, mobility, grip strength, vision and hearing, in addition to overall conditions such as the health of the coat and body temperature. The Has2 mice scored lower in frailty than the controls by all measures. They also performed better in tests of locomotion and coordination, and in bone density.
Gorbunova’s results show that a gene artificially transferred from one species can have a beneficial effect on another species for longevity, something that had never been demonstrated before. This finding is “quite spectacular,” said Steven Austad, a biologist at the University of Alabama at Birmingham, who was not involved in the study.
Just as in lifespan, the effects in various organs and systems varied between the sexes, a common occurrence in longevity research, according to Austad, who authored the book Methuselah’s Zoo and specializes in the biological differences between species. “We have ten drugs that we can give to mice to make them live longer,” he says, “and all of them work better in one sex than in the other.” This suggests that more attention needs to be paid to the different effects of anti-aging strategies between the sexes, as well as gender differences in healthspan.
According to the study authors, the HMM-HA molecule delivered these benefits by reducing inflammation and senescence (cell dysfunction and death). The molecule also caused a variety of other benefits, including an upregulation of genes involved in the function of mitochondria, the powerhouses of the cells. These mechanisms are implicated in the aging process, and in human disease. In humans, virtually all noncommunicable diseases entail an acceleration of the aging process.
So, would the gene that creates HMM-HA have similar benefits for longevity in humans? “We think about these questions a lot,” Gorbunova says. “It’s been done by injections in certain patients, but it has a local effect in the treatment of organs affected by disease,” which could offer some benefits, she added.
“Mice are very short-lived and cancer-prone, and the effects are small,” says Steven Austad, a biologist at the University of Alabama at Birmingham. “But they did live longer and stay healthy longer, which is remarkable.”
As for a gene therapy to introduce the nmrHas2 gene into humans to obtain a global result, she’s skeptical because of the complexity involved. Gorbunova notes that there are potential dangers in introducing an animal gene into humans, such as immune responses or allergic reactions.
Austad is equally cautious about a gene therapy. “What this study says is that you can take something a species does well and transfer at least some of that into a new species. It opens up the way, but you may need to transfer six or eight or ten genes into a human” to get the large effect desired. Humans are much more complex and contain many more genes than mice, and all systems in a biological organism are intricately connected. One naked mole rat gene may not make a big difference when it interacts with human genes, metabolism and physiology.
Still, Austad thinks the possibilities are tantalizing. “Mice are very short-lived and cancer-prone, and the effects are small,” he says. “But they did live longer and stay healthy longer, which is remarkable.”
As for further research, says Austad, “The first place to look is the skin” to see if the nmrHas2 gene and the HMM-HA it produces can reduce the chance of cancer. Austad added that it would be straightforward to use the gene to try to prevent cancer in skin cells in a dish to see if it prevents cancer. It would not be hard to do. “We don’t know of any downsides to hyaluronic acid in skin, because it’s already used in skin products, and you could look at this fairly quickly.”
“Aging mechanisms evolved over a long time,” says Gorbunova, “so in aging there are multiple mechanisms working together that affect each other.” All of these processes could play a part and almost certainly differ from one species to the next.
“HMM-HA molecules are large, but we’re now looking for a small-molecule drug that would slow it’s breakdown,” she says. “And we’re looking for inhibitors, now being tested in mice, that would hinder the breakdown of hyaluronic acid.” Gorbunova has found a natural, plant-based product that acts as an inhibitor and could potentially be taken as a supplement. Ultimately, though, she thinks that drug development will be the safest and most effective approach to delivering HMM-HA for anti-aging.
A new study provides key insights in what causes Alzheimer's: a breakdown in the brain’s system for clearing waste.
In recent years, researchers of Alzheimer’s have made progress in figuring out the complex factors that lead to the disease. Yet, the root cause, or causes, of Alzheimer’s are still pretty much a mystery.
In fact, many people get Alzheimer’s even though they lack the gene variant we know can play a role in the disease. This is a critical knowledge gap for research to address because the vast majority of Alzheimer’s patients don’t have this variant.
A new study provides key insights into what’s causing the disease. The research, published in Nature Communications, points to a breakdown over time in the brain’s system for clearing waste, an issue that seems to happen in some people as they get older.
Michael Glickman, a biologist at Technion – Israel Institute of Technology, helped lead this research. I asked him to tell me about his approach to studying how this breakdown occurs in the brain, and how he tested a treatment that has potential to fix the problem at its earliest stages.
Dr. Michael Glickman is internationally renowned for his research on the ubiquitin-proteasome system (UPS), the brain's system for clearing the waste that is involved in diseases such as Huntington's, Alzheimer's, and Parkinson's. He is the head of the Lab for Protein Characterization in the Faculty of Biology at the Technion – Israel Institute of Technology. In the lab, Michael and his team focus on protein recycling and the ubiquitin-proteasome system, which protects against serious diseases like Alzheimer’s, Parkinson’s, cystic fibrosis, and diabetes. After earning his PhD at the University of California at Berkeley in 1994, Michael joined the Technion as a Senior Lecturer in 1998 and has served as a full professor since 2009.

Dr. Michael Glickman

