A Cure for Sickle Cell Disease Is Coming. Will Patients Accept It?
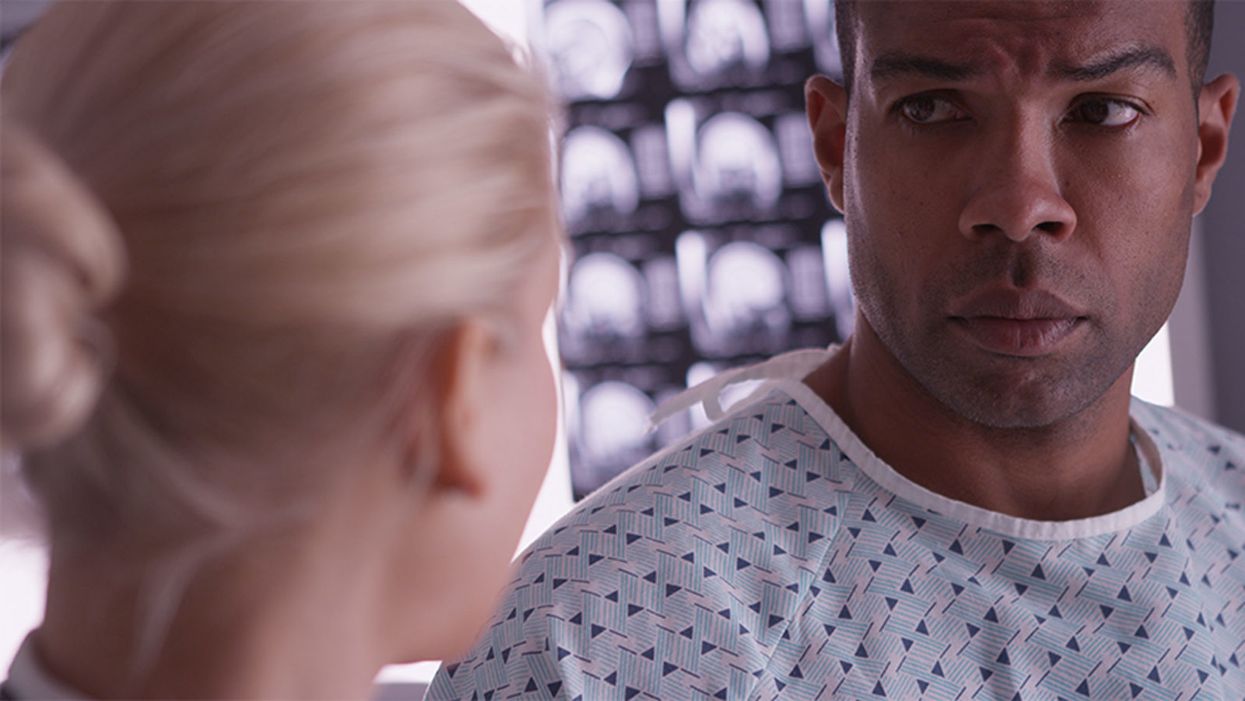
Many patients in the African-American community are skeptical of new experimental treatments, thanks to a history of medical exploitation in the 20th century.
If any malady proves the fragile grace of the human genome, it is sickle cell disease.
If experimental treatments receive regulatory approval, it would be a watershed breakthrough for tens of thousands of Americans.
It occurs because of a single "misspelled" letter of DNA, causing red blood cells to run low on oxygen and transforming the hemoglobin in each cell into a stiff rod. Normally round cells become rigid crescents that hamper the flow of blood throughout the body, like leaves clumping in a drain.
Strokes in toddlers are merely the beginning of the circulatory calamities this disease may inflict. Most sickled cells cannot carry oxygen through the body, causing anemia as well as excruciating chronic pain. Older patients are at risk of kidney failure, heart disease and all the other collateral damage caused by poor circulation. Few live beyond middle age.
The only way to cure it has been through a bone marrow transplant from a donor, which requires not only a closely matching volunteer, but bouts of chemotherapy to allow new stem cells to take root, as well as rounds of immunosuppressive drugs that may last for years.
Recent advances in genomic medicine may soon alter the disease's outlook, although many obstacles remain.
In one treatment under development, patient's skin cells are converted into stem cells, allowing them to be inserted into the bone marrow without the need for a donor. Another treatment known as gene therapy involves replacing the aberrant gene in the patient's body with new genetic material.
Although both remain in clinical trials -- and also require at least chemotherapy -- they have shown promise. Matthew Hsieh, a hematologist and staff scientist with the National Heart Lung and Blood Institute in Maryland, has performed about 10 gene therapy procedures over the past three years as part of a clinical trial. Ongoing tweaks in the procedure have led to the blood in more recent patients showing sickle cell trait -- not a perfect outcome, but one that leaves patients with far fewer symptoms than if they have the full-blown disease.
If one or both treatments receive regulatory approval, it would be a watershed breakthrough for the tens of thousands of Americans who suffer from the disease.
Yet it is entirely possible many patients may decline the cure.
A Painful History
The vast majority of sickle cell sufferers in the U.S. -- well beyond 90 percent -- are African-American, a population with a historically uneasy relationship toward healthcare.
"There is a lot of data on distrust between African-Americans and American medical institutions," says J. Corey Williams, a psychiatrist with the Children's Hospital of Philadelphia who has written extensively on racial disparities in healthcare. "It comes from a long legacy of feeling victimized by medicine."
"What you hear from many patients is 'I am not going to be your guinea pig, and I am not going to be experimented on.'"
As a result, Williams is among several clinicians interviewed for this story who believe a cure for sickle cell disease would be embraced reluctantly.
"What you hear from many patients is 'I am not going to be your guinea pig, and I am not going to be experimented on.' And so the history of African-Americans and research will manifest as we develop gene therapies for [these] patients," says Christopher L. Edwards, a clinical psychologist and researcher with the Maya Angelou Center for Health Equity at the Wake Forest University School of Medicine.
Fear among African-Americans of becoming guinea pigs is well-founded. The first c-sections and fistula repairs occurring in North America were performed on enslaved women -- all without consent and virtually none with anesthesia.
Modern 20th century medicine led to the Tuskegee syphilis experiments conducted by the U.S. Public Health Service. Researchers withheld treatment from some 400 African-American men from the 1930s well into the 1970s to observe how they reacted to the disease -- even though curative antibiotics had been around for decades. Only news reports ended the experiment.
The long-standing distrust of American healthcare in the African-American community is also baked into the care provided to sickle cell patients. Despite affecting one in 365 African-Americans, there is no disease registry to assist clinical trials, according to Mary Hulihan, a blood disorders epidemiologist with the Centers for Disease Control and Prevention. Edwards says many sufferers are suspicious of being monitored.
Meanwhile, only two drugs are available to alleviate the worst symptoms. The first one, hydroxyurea, received FDA approval only in 1998 -- nearly 90 years after the disease was first diagnosed. Moreover, Edwards says that some sufferers shy away from using hydroxyurea because it is also used to treat cancer. It's part of what he calls the "myth and folklore" in the African-American community about sickle cell disease.
Economics plays a role as well in the often-fragmented care such patients receive. According to CDC data, many patients rely extensively on public insurance programs such as Medicaid, whose coverage varies from state to state.
A Tough Transition
Edwards notes that sickle cell sufferers usually receive good care when they're children because of support provided by family members. But that often breaks down in adulthood. According to CDC data, an adult sickle cell patient visits a hospital emergency room three times as often as a child patient.
The consensus is that the path to a medical cure for sickle cell will first need to be smoothed over with a talk cure.
Modupe Idowu, a hematologist with the University of Texas Health system, estimates that there are perhaps a dozen comprehensive care centers for the estimated 100,000 sickle cell patients in the U.S., including the one she operates in Houston. That means a significant proportion of those afflicted are on their own to procure care.
And since many patients are on Medicaid, "a lot of hematologists that train to take care of blood disorders, many are not interested in treating [sickle cell disease] because the reimbursement for providers is not great," Idowu says.
Hsieh acknowledges that many of his patients can be suspicious about the care they are receiving. Frustration with fragmented care is usually the biggest driver, he adds.
Meanwhile, the skepticism that patients have about the treatments they seek is often reciprocated by their caregivers.
"The patients have experiences with medication and know what works at a very young age (for their pain)," Edwards says. Such expertise demonstrated by an African-American patient often leads to them being labeled as narcotics seekers.
The Correct Path
This all begs the question of how to deploy a cure. Idowu, who regularly holds town hall-style meetings with Houston-area patients, often must allay anxieties. For example, the gene therapy approach uses a harmless virus to transport new genetic material into cells. That virus happens to be a benign version of HIV, and convincing patients they won't be infected with HIV is a fraught issue.
The consensus is that the path to a medical cure for sickle cell will first need to be smoothed over with a talk cure.
Idowu tries to hammer home the fact that patients are afforded vastly more protections than in the past. "There are a lot of committees and investigational review boards that keep track of clinical trials; things just don't happen anymore as they did in the past," she says. She also believes it helps if more providers of color communicate to patients.
Hsieh is very straightforward with his patients. He informs them about the HIV vector but assures them no one has ever tested positive for the virus as a result of its use.
Edwards notes that since many patients suffer psychosocial trauma as a result of their chronic pain, there already is some counseling infrastructure in place to help them cope. He believes such resources will have to be stretched further as a cure looms closer.
In the absence of formal mental health services, straight talk may be the best way to overcome wariness.
"If patients have misgivings, we try our best to address them, and let them know at the end of the day it is their decision to make," Hsieh says. "And even the patients who have gone through the gene therapy and it didn't work well -- they're still glad they took the chance."
The future of non-hormonal birth control: Antibodies can stop sperm in their tracks
Many women want non-hormonal birth control. A 22-year-old's findings were used to launch a company that could, within the decade, bring a new kind of contraceptive to the marketplace.
Unwanted pregnancy can now be added to the list of preventions that antibodies may be fighting in the near future. For decades, really since the 1980s, engineered monoclonal antibodies have been knocking out invading germs — preventing everything from cancer to COVID. Sperm, which have some of the same properties as germs, may be next.
Not only is there an unmet need on the market for alternatives to hormonal contraceptives, the genesis for the original research was personal for the then 22-year-old scientist who led it. Her findings were used to launch a company that could, within the decade, bring a new kind of contraceptive to the marketplace.
The genesis
It’s Suruchi Shrestha’s research — published in Science Translational Medicine in August 2021 and conducted as part of her dissertation while she was a graduate student at the University of North Carolina at Chapel Hill — that could change the future of contraception for many women worldwide. According to a Guttmacher Institute report, in the U.S. alone, there were 46 million sexually active women of reproductive age (15–49) who did not want to get pregnant in 2018. With the overturning of Roe v. Wade last year, Shrestha’s research could, indeed, be life changing for millions of American women and their families.
Now a scientist with NextVivo, Shrestha is not directly involved in the development of the contraceptive that is based on her research. But, back in 2016 when she was going through her own problems with hormonal contraceptives, she “was very personally invested” in her research project, Shrestha says. She was coping with a long list of negative effects from an implanted hormonal IUD. According to the Mayo Clinic, those can include severe pelvic pain, headaches, acute acne, breast tenderness, irregular bleeding and mood swings. After a year, she had the IUD removed, but it took another full year before all the side effects finally subsided; she also watched her sister suffer the “same tribulations” after trying a hormonal IUD, she says.
For contraceptive use either daily or monthly, Shrestha says, “You want the antibody to be very potent and also cheap.” That was her goal when she launched her study.
Shrestha unshelved antibody research that had been sitting idle for decades. It was in the late 80s that scientists in Japan first tried to develop anti-sperm antibodies for contraceptive use. But, 35 years ago, “Antibody production had not been streamlined as it is now, so antibodies were very expensive,” Shrestha explains. So, they shifted away from birth control, opting to focus on developing antibodies for vaccines.
Over the course of the last three decades, different teams of researchers have been working to make the antibody more effective, bringing the cost down, though it’s still expensive, according to Shrestha. For contraceptive use either daily or monthly, she says, “You want the antibody to be very potent and also cheap.” That was her goal when she launched her study.
The problem
The problem with contraceptives for women, Shrestha says, is that all but a few of them are hormone-based or have other negative side effects. In fact, some studies and reports show that millions of women risk unintended pregnancy because of medical contraindications with hormone-based contraceptives or to avoid the risks and side effects. While there are about a dozen contraceptive choices for women, there are two for men: the condom, considered 98% effective if used correctly, and vasectomy, 99% effective. Neither of these choices are hormone-based.
On the non-hormonal side for women, there is the diaphragm which is considered only 87 percent effective. It works better with the addition of spermicides — Nonoxynol-9, or N-9 — however, they are detergents; they not only kill the sperm, they also erode the vaginal epithelium. And, there’s the non-hormonal IUD which is 99% effective. However, the IUD needs to be inserted by a medical professional, and it has a number of negative side effects, including painful cramping at a higher frequency and extremely heavy or “abnormal” and unpredictable menstrual flows.
The hormonal version of the IUD, also considered 99% effective, is the one Shrestha used which caused her two years of pain. Of course, there’s the pill, which needs to be taken daily, and the birth control ring which is worn 24/7. Both cause side effects similar to the other hormonal contraceptives on the market. The ring is considered 93% effective mostly because of user error; the pill is considered 99% effective if taken correctly.
“That’s where we saw this opening or gap for women. We want a safe, non-hormonal contraceptive,” Shrestha says. Compounding the lack of good choices, is poor access to quality sex education and family planning information, according to the non-profit Urban Institute. A focus group survey suggested that the sex education women received “often lacked substance, leaving them feeling unprepared to make smart decisions about their sexual health and safety,” wrote the authors of the Urban Institute report. In fact, nearly half (45%, or 2.8 million) of the pregnancies that occur each year in the US are unintended, reports the Guttmacher Institute. Globally the numbers are similar. According to a new report by the United Nations, each year there are 121 million unintended pregnancies, worldwide.
The science
The early work on antibodies as a contraceptive had been inspired by women with infertility. It turns out that 9 to 12 percent of women who are treated for infertility have antibodies that develop naturally and work against sperm. Shrestha was encouraged that the antibodies were specific to the target — sperm — and therefore “very safe to use in women.” She aimed to make the antibodies more stable, more effective and less expensive so they could be more easily manufactured.
Since antibodies tend to stick to things that you tell them to stick to, the idea was, basically, to engineer antibodies to stick to sperm so they would stop swimming. Shrestha and her colleagues took the binding arm of an antibody that they’d isolated from an infertile woman. Then, targeting a unique surface antigen present on human sperm, they engineered a panel of antibodies with as many as six to 10 binding arms — “almost like tongs with prongs on the tongs, that bind the sperm,” explains Shrestha. “We decided to add those grabbers on top of it, behind it. So it went from having two prongs to almost 10. And the whole goal was to have so many arms binding the sperm that it clumps it” into a “dollop,” explains Shrestha, who earned a patent on her research.

Suruchi Shrestha works in the lab with a colleague. In 2016, her research on antibodies for birth control was inspired by her own experience with side effects from an implanted hormonal IUD.
UNC - Chapel Hill
The sperm stays right where it met the antibody, never reaching the egg for fertilization. Eventually, and naturally, “Our vaginal system will just flush it out,” Shrestha explains.
“She showed in her early studies that [she] definitely got the sperm immotile, so they didn't move. And that was a really promising start,” says Jasmine Edelstein, a scientist with an expertise in antibody engineering who was not involved in this research. Shrestha’s team at UNC reproduced the effect in the sheep, notes Edelstein, who works at the startup Be Biopharma. In fact, Shrestha’s anti-sperm antibodies that caused the sperm to agglutinate, or clump together, were 99.9% effective when delivered topically to the sheep’s reproductive tracts.
The future
Going forward, Shrestha thinks the ideal approach would be delivering the antibodies through a vaginal ring. “We want to use it at the source of the spark,” Shrestha says, as opposed to less direct methods, such as taking a pill. The ring would dissolve after one month, she explains, “and then you get another one.”
Engineered to have a long shelf life, the anti-sperm antibody ring could be purchased without a prescription, and women could insert it themselves, without a doctor. “That's our hope, so that it is accessible,” Shrestha says. “Anybody can just go and grab it and not worry about pregnancy or unintended pregnancy.”
Her patented research has been licensed by several biotech companies for clinical trials. A number of Shrestha’s co-authors, including her lab advisor, Sam Lai, have launched a company, Mucommune, to continue developing the contraceptives based on these antibodies.
And, results from a small clinical trial run by researchers at Boston University Chobanian & Avedisian School of Medicine show that a dissolvable vaginal film with antibodies was safe when tested on healthy women of reproductive age. That same group of researchers last year received a $7.2 million grant from the National Institute of Health for further research on monoclonal antibody-based contraceptives, which have also been shown to block transmission of viruses, like HIV.
“As the costs come down, this becomes a more realistic option potentially for women,” says Edelstein. “The impact could be tremendous.”
This article was first published by Leaps.org in December, 2022. It has been lightly edited with updates for timeliness.
Researchers probe extreme gene therapy for severe alcoholism
When all traditional therapeutic approaches fail for alcohol abuse disorder, a radical gene therapy might be something to try in the future.
Story by Freethink
A single shot — a gene therapy injected into the brain — dramatically reduced alcohol consumption in monkeys that previously drank heavily. If the therapy is safe and effective in people, it might one day be a permanent treatment for alcoholism for people with no other options.
The challenge: Alcohol use disorder (AUD) means a person has trouble controlling their alcohol consumption, even when it is negatively affecting their life, job, or health.
In the U.S., more than 10 percent of people over the age of 12 are estimated to have AUD, and while medications, counseling, or sheer willpower can help some stop drinking, staying sober can be a huge struggle — an estimated 40-60 percent of people relapse at least once.
A team of U.S. researchers suspected that an in-development gene therapy for Parkinson’s disease might work as a dopamine-replenishing treatment for alcoholism, too.
According to the CDC, more than 140,000 Americans are dying each year from alcohol-related causes, and the rate of deaths has been rising for years, especially during the pandemic.
The idea: For occasional drinkers, alcohol causes the brain to release more dopamine, a chemical that makes you feel good. Chronic alcohol use, however, causes the brain to produce, and process, less dopamine, and this persistent dopamine deficit has been linked to alcohol relapse.
There is currently no way to reverse the changes in the brain brought about by AUD, but a team of U.S. researchers suspected that an in-development gene therapy for Parkinson’s disease might work as a dopamine-replenishing treatment for alcoholism, too.
To find out, they tested it in heavy-drinking monkeys — and the animals’ alcohol consumption dropped by 90% over the course of a year.
How it works: The treatment centers on the protein GDNF (“glial cell line-derived neurotrophic factor”), which supports the survival of certain neurons, including ones linked to dopamine.
For the new study, a harmless virus was used to deliver the gene that codes for GDNF into the brains of four monkeys that, when they had the option, drank heavily — the amount of ethanol-infused water they consumed would be equivalent to a person having nine drinks per day.
“We targeted the cell bodies that produce dopamine with this gene to increase dopamine synthesis, thereby replenishing or restoring what chronic drinking has taken away,” said co-lead researcher Kathleen Grant.
To serve as controls, another four heavy-drinking monkeys underwent the same procedure, but with a saline solution delivered instead of the gene therapy.
The results: All of the monkeys had their access to alcohol removed for two months following the surgery. When it was then reintroduced for four weeks, the heavy drinkers consumed 50 percent less compared to the control group.
When the researchers examined the monkeys’ brains at the end of the study, they were able to confirm that dopamine levels had been replenished in the treated animals, but remained low in the controls.
The researchers then took the alcohol away for another four weeks, before giving it back for four. They repeated this cycle for a year, and by the end of it, the treated monkeys’ consumption had fallen by more than 90 percent compared to the controls.
“Drinking went down to almost zero,” said Grant. “For months on end, these animals would choose to drink water and just avoid drinking alcohol altogether. They decreased their drinking to the point that it was so low we didn’t record a blood-alcohol level.”
When the researchers examined the monkeys’ brains at the end of the study, they were able to confirm that dopamine levels had been replenished in the treated animals, but remained low in the controls.
Looking ahead: Dopamine is involved in a lot more than addiction, so more research is needed to not only see if the results translate to people but whether the gene therapy leads to any unwanted changes to mood or behavior.
Because the therapy requires invasive brain surgery and is likely irreversible, it’s unlikely to ever become a common treatment for alcoholism — but it could one day be the only thing standing between people with severe AUD and death.
“[The treatment] would be most appropriate for people who have already shown that all our normal therapeutic approaches do not work for them,” said Grant. “They are likely to create severe harm or kill themselves or others due to their drinking.”
This article originally appeared on Freethink, home of the brightest minds and biggest ideas of all time.


