A New Test Aims to Objectively Measure Pain. It Could Help Legitimate Sufferers Access the Meds They Need.
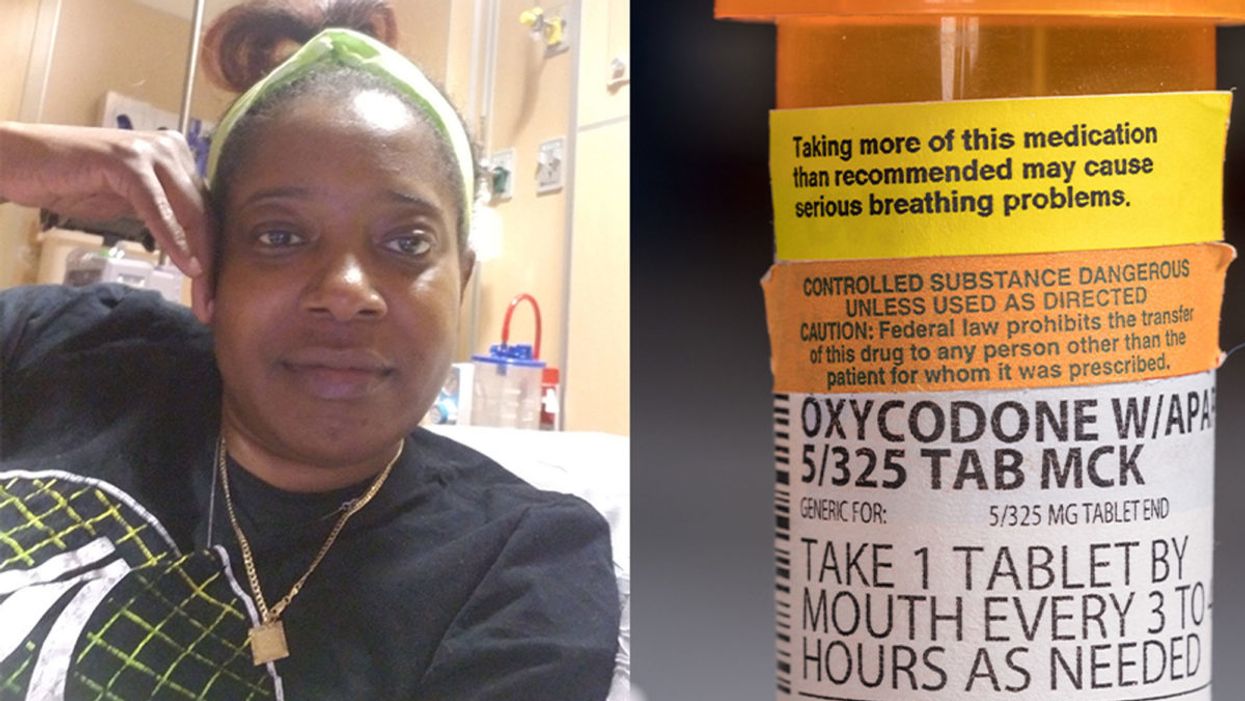
Sickle cell patient Bridgett Willkie found herself being labeled an addict when she sought an opioid prescription to control her pain.
"That throbbing you feel for the first minute after a door slams on your finger."
This is how Central Florida resident Bridgett Willkie describes the attacks of pain caused by her sickle cell anemia – a genetic blood disorder in which a patient's red blood cells become shaped like sickles and get stuck in blood vessels, thereby obstructing the flow of blood and oxygen.
"I found myself being labeled as an addict and I never was."
Willkie's lifelong battle with the condition has led to avascular necrosis in both of her shoulders, hips, knees and ankles. This means that her bone tissue is dying due to insufficient blood supply (sickle cell anemia is among the medical conditions that can decrease blood flow to one's bones).
"That adds to the pain significantly," she says. "Every time my heart beats, it hurts. And the pain moves. It follows the path of circulation. I liken it to a traffic jam in my veins."
For more than a decade, she received prescriptions for Oxycontin. Then, four years ago, her hematologist – who had been her doctor for 18 years – suffered a fatal heart attack. She says her longtime doctor's replacement lacked experience treating sickle cell patients and was uncomfortable writing her a prescription for opioids. What's more, this new doctor wanted to place her in a drug rehab facility.
"Because I refused to go, he stopped writing my scripts," she says. The ensuing three months were spent at home, detoxing. She describes the pain as unbearable. "Sometimes I just wanted to die."
One of the effects of the opioid epidemic is that many legitimate pain patients have seen their opioids significantly reduced or downright discontinued because of their doctors' fears of over-prescribing addictive medications.
"I found myself being labeled as an addict and I never was...Being treated like a drug-seeking patient is degrading and humiliating," says Willkie, who adds that when she is at the hospital, "it's exhausting arguing with the doctors...You dread them making their rounds because every day they come in talking about weaning you off your meds."
Situations such as these are fraught with tension between patients and doctors, who must remain wary about the risk of over-prescribing powerful and addictive medications. Adding to the complexity is that it can be very difficult to reliably assess a patient's level of physical pain.
However, this difficulty may soon decline, as Indiana University School of Medicine researchers, led by Dr. Alexander B. Niculescu, have reportedly devised a way to objectively assess physical pain by analyzing biomarkers in a patient's blood sample. The results of a study involving more than 300 participants were published earlier this year in the journal Molecular Psychiatry.
Niculescu – who is both a professor of psychiatry and medical neuroscience at the IU School of Medicine – explains that, when someone is in severe physical pain, a blood sample will show biomarkers related to intracellular adhesion and cell-signaling mechanisms. He adds that some of these biomarkers "have prior convergent evidence from animal or human studies for involvement in pain."
Aside from reliably measuring pain severity, Niculescu says blood biomarkers can measure the degree of one's response to treatment and also assess the risk of future recurrences of pain. He believes this new method's greatest benefit, however, might be the ability to identify a number of non-opioid medications that a particular patient is likely to respond to, based on his or her biomarker profile.
Clearly, such a method could be a gamechanger for pain patients and the professionals who treat them. As of yet, health workers have been forced to make crucial decisions based on their clinical impressions of patients; such impressions are invariably subjective. A method that enables people to prove the extent of their pain could remove the stigma that many legitimate pain patients face when seeking to obtain their needed medicine. It would also improve their chances of receiving sufficient treatment.
Niculescu says it's "theoretically possible" that there are some conditions which, despite being severe, might not reveal themselves through his testing method. But he also says that, "even if the same molecular markers that are involved in the pain process are not reflected in the blood, there are other indirect markers that should reflect the distress."
Niculescu expects his testing method will be available to the medical community at large within one to three years.
Willkie says she would welcome a reliable pain assessment method. Well-aware that she is not alone in her plight, she has more than 500 Facebook friends with sickle cell disease, and she says that "all of their opioid meds have been restricted or cut" as a result of the opioid crisis. Some now feel compelled to find their opioids "on the streets." She says she personally has never obtained opioids this way. Instead, she relies on marijuana to mitigate her pain.
Niculescu expects his testing method will be available to the medical community at large within one to three years: "It takes a while for things to translate from a lab setting to a commercial testing arena."
In the meantime, for Willkie and other patients, "we have to convince doctors and nurses that we're in pain."
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained

Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

