A Star Surgeon Left a Trail of Dead Patients—and His Whistleblowers Were Punished

Dr. Paolo Macchiarini, then a professor of Regenerative Medicine at Karolinska Institute in Stockholm (Sweden), looks on during a plenary session at the Science of the Future international scientific conference, on September 17, 2014.
[Editor's Note: This is the first comprehensive account of the whistleblowers' side of a scandal that rocked the most hallowed halls in science – the same establishment that just last week awarded the Nobel Prize in Medicine. This still-unfolding saga is a cautionary tale about corruption, hype, and power that raises profound questions about how to uphold integrity in scientific research.]
When the world-famous Karolinska Institutet (KI) in Stockholm hired Dr. Paolo Macchiarini, he was considered a star surgeon and groundbreaking stem cell researcher. Handsome, charming and charismatic, Macchiarini was known as a trailblazer in a field that holds hope for curing a vast array of diseases.
It appeared that Macchiarini's miracle cure was working just as expected.
He claimed that he was regenerating human windpipes by seeding plastic scaffolds with stem cells from the patient's own bone marrow—a holy grail in medicine because the body will not reject its own cells. For patients who had trouble breathing due to advanced illness, a trachea made of their own cells would be a game-changer. Supposedly, the bone marrow cells repopulated the synthetic scaffolds with functioning, mucus-secreting epithelial cells, creating a new trachea that would become integrated into the patient's respiratory system as a living, breathing part. Macchiarini said as much in a dazzling presentation to his new colleagues at Karolinska, which is home to the Nobel Assembly – the body that has awarded the Nobel Prizes in Physiology or Medicine since 1901.
Karl-Henrik Grinnemo was a young cardiothoracic surgeon and researcher at Karolinska in 2010, when Macchiarini was hired. "He gave a fantastic presentation with lots of animation and everyone was impressed," Grinnemo says of his first encounter with Macchiarini. Grinnemo's own work focused on heart and aortic valve regeneration, also in the field of stem cell research. He and his colleagues were to help establish an interdisciplinary umbrella organization, under Macchiarini's leadership, called the Advanced Center for Translational Regenerative Medicine, which would aim to deliver cures from Karolinska's world-class laboratories to the bedsides of patients in desperate need.
Little did Grinnemo know that when KI hired Macchiarini, they had ignored a warning that the star surgeon had been accused of scientific misconduct by a colleague who had worked with him at the University of Florence. That blind eye would eventually cost three patients their lives in Sweden.
"A MIRACLE CURE"?
It has been said that if all you have is a hammer, everything looks like a nail, and it wasn't long before Macchiarini announced that he had a patient in dire need of one of the new artificial tracheas. The patient, a native of Eritrea who had emigrated to Iceland, had a slowly growing tumor on his trachea. Macchiarini had previously generated new windpipes from human donor tracheas outside of Sweden, but the Icelandic patient was the first to receive a synthetic trachea implant at Karolinska University Hospital. Macchiarini had already performed a similar procedure with decellularized donor tracheas on other patients around Europe, but not much was known at the time about their outcomes.
Of course, to justify a radical procedure such as removing a patient's trachea, one would need compelling evidence of effectiveness in animal studies, as well as an exhaustion of all other treatment alternatives. Macchiarini claimed that both conditions were met. He performed the implantation of the synthetic trachea as if he had received a hospital exemption. This is comparable to what the U.S. Food and Drug Administration classifies as "compassionate use," a procedure performed only in extreme circumstances, usually when the patient is terminal, and when no available alternative has worked.
Macchiarini personally invited Grinnemo to watch the all-day surgery, and, once the transplant was done after 10 grueling hours, Macchiarini asked him to close the patient. Then the 36-year-old man was transferred to another hospital, where Grinnemo and other attending physicians had little opportunity to follow his long-term recovery.
Two months later, Macchiarini approached Grinnemo with an invitation to be one of multiple co-authors on a paper about the case targeted for the New England Journal of Medicine. This was a huge opportunity for a junior researcher, and Grinnemo gladly agreed to write a one-month follow-up report on the Icelandic patient's clinical condition. He consulted the patient's medical records, which described a man with an infection in one lung but otherwise doing well, and wrote up his contribution. The patient had already been transferred back to Iceland by then and was home from the hospital. It appeared that Macchiarini's miracle cure was working just as expected.
But the ground was beginning to shake.
"We cannot find one word of evidence that points to regeneration induced by stem cells."
On September 2, 2011, three months after the Icelandic patient's surgery, a professor in Leuven, Belgium sent a written warning to KI's vice chancellor, Harriett Wallberg-Henriksson, stating that Macchiarini was guilty of prior research misconduct. This letter was forwarded to the new president at KI, professor Anders Hamsten, urging him to put a halt to more synthetic trachea implants. The accusations were grave.
Professor Pierre Delaere at Kathiolieke Universiteit asserted that synthetic tracheas coated with bone marrow cells did not, as Macchiarini had claimed, transform into living tracheas. He cited "countless" failures in animal experiments and called the outcome of Macchiarini's previous human surgeries "disastrous…half the patients died. The others are in a palliative setting….We cannot find one word of evidence that points to regeneration induced by stem cells."
Once again, KI simply ignored the warning, and Grinnemo and the 24 co-authors on the splashy academic paper about the latest surgery didn't even know about it. In the meantime, the New England Journal of Medicine rejected it for lacking a longer follow-up on the patients and missing data on how well the implants had integrated with the patient's respiratory system, so Macchiarini submitted it to The Lancet instead.
And he kept performing his experimental surgeries.
Soon there was a second transplant patient, a 30-year-old American man named Christopher Lyles. After his operation at KI, he returned to the U.S and the Swedish doctors were unable to follow his progress. Three months after his surgery, they learned that he had died at his home.
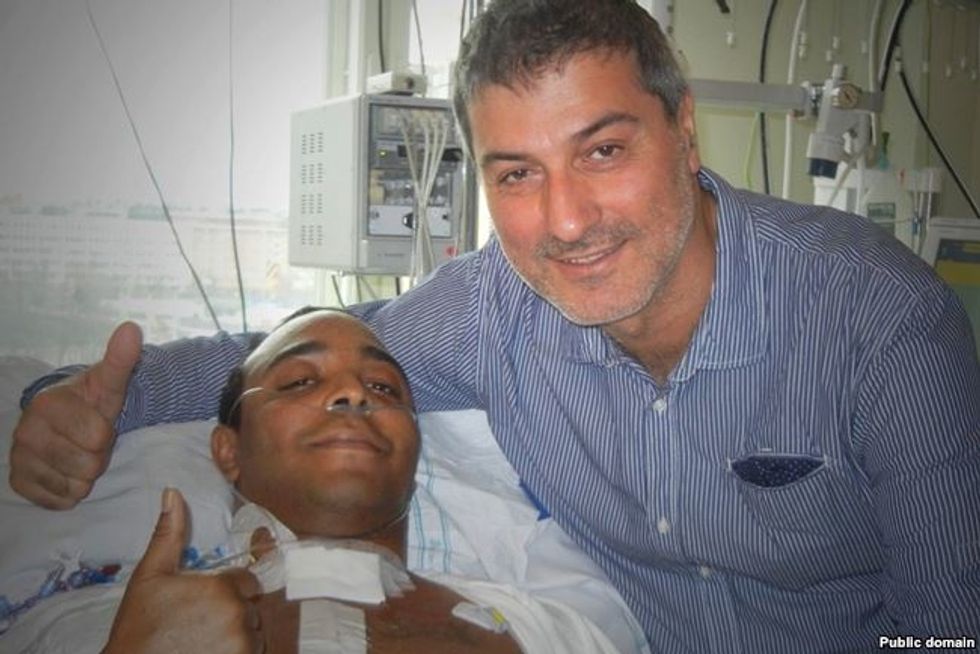
Paolo Macchiarini with Christopher Lyles, the American patient on whom he performed a trachea transplant in Stockholm in 2011. Lyles died a few months later.
Only four months after Lyles died, the third patient, a 22-year-old Turkish woman, received one of Macchiarini's grafts. In all three patients, Macchiarini had claimed that they were in dire straights—terminal if not for the hope of a trachea transplant, and he claimed a hospital exemption in all three cases. In fact, Grinnemo says, all three had been in stable condition before their surgeries—a reality Macchiarini did not share with his collaborators and co-authors on two academic papers about the surgeries that were subsequently published in The Lancet.
The Turkish woman's story is especially tragic. The young woman had initially undergone surgery elsewhere to fix an unrelated problem—hand sweating--but wound up with an accidentally damaged trachea that set her on a course of utter devastation. She sought help from Macchiarini, but his graft operation left her "living in hell," says Grinnemo. In intensive care afterward, her airways were producing so much mucus that they had to be cleared every four hours around the clock. The procedure "is like someone keeping your head under water every fourth hour until you almost suffocate to death. This is something that you wouldn't wish on your worst enemy," says Grinnemo.
By the spring of 2013, six months after Macchiarini's operation, the graft began to collapse. Several metal stents were inserted into her airways, but each one only worked for a short while. Macchiarini decided to remove the first plastic trachea and implant a new one. It seemed she couldn't get any worse, but after the second transplant, the young woman further deteriorated. Her airway secretions only increased; she had to undergo thousands of bronchoscopies, where an instrument was pushed down her throat into her lungs, and hundreds of surgeries during her three-year stint in the intensive care unit. Her body couldn't tolerate much more.
The whistleblowers realized that, despite Macchiarini's claims of successful operations in several now-published papers, the patients had been mutilated.
Grinnemo, together with fellow KI physicians Matthias Corbascio, Oscar Simonson and Thomas Fux, who were all involved in the care of the Turkish woman, became alarmed when the Icelandic patient came back to their hospital in the fall of 2013 with similar complaints. They realized that, despite Macchiarini's claims of successful operations in several now-published papers, the patients had been mutilated.
Both the Icelandic patient and the Turkish woman were too incapacitated to speak for themselves, so in the late fall of 2013, Grinnemo and his three concerned colleagues reached out to the patients' relatives seeking permission to review their medical records. It took weeks to receive the permissions, but once they did, what they found stunned them.
The Icelandic patient had developed fistulas (holes) between the artificial trachea and his esophagus, and had been fitted with several stents. Soon his esophagus also had to be removed, which Macchiarini was aware of. He should have reported these complications in the articles on which he was lead author, Grinnemo contends, and also should have informed his co-authors, each of whom had been responsible for writing up discrete sections of the papers. But Macchiarini had described each transplant as a success and had greatly exaggerated, if not outright lied, about how each patient had fared.
THE WHISTLEBLOWERS FIGHT BACK
Grinnemo and several other suspicious colleagues decided to launch an investigation. The result was a 500-page report identifying the synthetic tracheas as the problem and revealing that Macchiarini had falsified data and suppressed critical information in his reporting. He had even invented biopsies of the grafts, claiming that the marrow cells had populated them with functioning epithelial cells, while there was no real evidence of the patients' cells growing to line the tracheas.
The whistleblowers also discovered that Macchiarini had never received ethical clearance from Sweden's Human Ethical Review Board, nor had he gotten approval for his plastic tracheas from the Medical Product Agency, the Swedish counterpart to the FDA. He had relied entirely on his ability to do the surgeries under the hospital exemption, which he made everyone believe that he had obtained thanks to his star power.
What Macchiarini was doing, the investigators realized, was experimentation on living human subjects; he had circumvented the normal oversight protocols that exist to protect such subjects.
At a procedural meeting with his colleagues, including Dr. Ulf Lockowandt, the head of Karolinska University Hospital's Department of Cardiothoracic Surgery, Macchiarini dismissed the patients' complications as "manageable."
But among the large interdisciplinary team whose members had knowledge only of their own discrete specialties, doubts about Macchiarini's technique were festering. Complications in the patients only worsened when the tracheas inevitably began to collapse. There was a bursting open of sutures, holes in tissues adjacent to the implants, the disintegration of tissues that clogged bronchial passages. In far more than half of all the patients Macchiarini had operated on in several countries, patients died a lingering and agonizing death.
The last thing the whistleblowers expected was for the full weight of the institution to come crashing down against them.
When Grinnemo and his fellow investigators dug all this up, they decided they had to report it to the very top of Karolinska, to the institute's president, Anders Hamsten, so that he could stop Macchiarini from performing any further transplants. The last thing the whistleblowers expected was for the full weight of the institution to come crashing down against them.
"THEY WANTED TO SILENCE EVERYTHING"
KI had ample reason to sweep criticisms of Macchiarini under the rug. Up to 100 patients were about to be recruited for an international clinical study in which Macchiarini would do his implants—a nightmarish prospect considering his track record. But KI stood to receive millions of dollars in a government grant to conduct the study across Europe and Russia.
Still other incentives existed for KI to suppress Macchiarini's record. Plans were underway to establish a stem cell center in Hong Kong with over $45 million provided by a wealthy Chinese businessman. At the center, Macchiarini would be able to do his trachea transplants on patients in Asia. And in addition to the financial incentives to keep Macchiarini's brand associated with KI, many high-powered individuals were involved in his initial recruitment and didn't want their reputations tarnished, Grinnemo says. KI not only ignored the whistleblowers' allegations; punishment against them was swift and decisive.
On March 7, 2014, Grinnemo and the other whistleblowers met with Dr. Hamsten, in addition to two of Macchiarini's supervisors and the director of KI's Regenerative Network. They presented their findings and requested an official investigation by KI, including scrutiny of the now-six published research papers in which Macchiarini had claimed the success of his implants in humans. The whistleblowers also told the leadership about some rat studies Macchiarini had published in a prestigious journal that appeared to rely on falsified data.
Instead of the welcoming reception they expected, the room bristled with hostility. "I basically forced them to agree to an investigation," Grinnemo says, "but it was a very tough meeting. The feeling I got was that they wanted to silence everything and that they would continue to silence me and the other whistleblowers. We were already feeling the backlash."

From the left, whistleblowers Matthias Corbascio, Oscar Simonson, Thomas Fux and Karl-Henrik Grinnemo.
Previously, Grinnemo had confronted Macchiarini with questions about patients he had implanted in Russia prior to his stint at Karolinska. "Paolo Macchiarini realized we were onto something and he became very angry. He said he would do everything in his power to make my life miserable," Grinnemo recalls.
Macchiarini made good on his threat by filing a complaint about Grinnemo with the Swedish Research Council, the main funder of research in Sweden. At the time, Macchiarini and Grinnemo had jointly submitted a grant application on an aortic valve regeneration project, which the Council had approved. Macchiarini suddenly complained that Grinnemo had stolen his data on aortic valve regeneration, even though, unlike Grinnemo, Macchiarini was not a heart surgeon and had conducted no research on heart structures. In reality, all of the data had been generated by Grinnemo. The Council did a review and concluded that Grinnemo had not stolen the data, but Macchiarini spread rumors throughout KI that the young researcher was guilty of scientific misconduct. "He wanted to discredit me because he knew I was dangerous and he wanted to stop anyone from believing me," Grinnemo says.
In spite of the findings from the Council that he had committed no scientific misconduct, KI opened an investigation—not of Macchiarini, but of Grinnemo himself. It soon became clear that KI also wanted to discredit Grinnemo and to silence any possible rumors about Macchiarini's conduct. The whistleblowers continued to push forward, however, and over a period of several weeks they wrote to president Hamsten four times, asking that KI investigate the deadly transplants still being promoted by Macchiarini as some kind of miracle cure.
After four written requests, Hamsten replied that if the whistleblowers had concerns about Macchiarini, they should contact their supervisors or write a formal complaint. But the whistleblowers had already contacted several individuals in supervisory roles who had made it clear that they wanted nothing to do with the affair. It was obvious that KI would resist any investigation of Macchiarini and that no one, outside of the whistleblowers, wanted to take any responsibility for what could amount to a major scandal at one of the world's most powerful academic institutions.
The whistleblowers had another hostile and unproductive meeting with several doctors at KI with whom they shared a letter they had written to the journal Nature Communications, which published Macchiarini's article on rat experimentation, urging them to investigate whether he had falsified the data. Once again, the whistleblowers met with a wall of resistance. Grinnemo was now discredited because of the aortic valve grant application, the doctors reminded him, and no investigation or retraction of the Nature Communications article would be pursued.
In June 2014, KI made its retaliation against Grinnemo official by putting its legal counsel in charge of its investigation of his grant application. The university's ethical board then concluded that Grinnemo should have informed Macchiarini more clearly that he submitted the application to the Swedish Research Council and that he should have obtained a written acceptance from Macchiarini before proceeding with the application. KI could not find Grinnemo guilty of research misconduct, but accused him of "carelessness" regarding the usage of data—which was his own data all along.
A few years later, Grinnemo was totally cleared by both the Central Ethical Review Board and KI. However, the rumors surrounding the investigation and the finding that he hadn't "used data correctly" in a grant application had done their damage to his reputation. Since then, he has not received a single research grant. "You can't appeal the findings," Grinnemo says. "I don't know if I will ever get more research money. I'm totally dead."
The whistleblowers made multiple appeals to Dr. Lockowandt, the head of the Department of Cardiothoracic surgery, for an investigation into Macchiarini's implants, but they were stonewalled from the beginning. Lockowandt did nothing.
"The heads of departments at the KUH and KI didn't actually have that much power," Grinnemo explains. "Dr. Lockowandt thought he was fighting for his own career and position. He's basically a good person who decided to go the route of an administrator, and if you have conflicts with your superiors, your career will be over." In other words, a real investigation of Macchiarini's record could not happen with so much money and prestige riding on the continued presence of the star surgeon.
By August 11, 2014, the whistleblowers had made repeated requests of Dr. Hamsten for a meeting to present the data inconsistencies between Macchiarini's patients' medical records and what he had reported in numerous articles, all published in prestigious medical journals. When they finally received the answer—a cold instruction to submit a written notification to the heads of their departments—it was clear that KI was giving them the runaround.
But rather than simply ignore the whistleblowers, KI apparently decided to double down, trying to discredit them in an intimidation campaign.
KI even went so far as to force the chief medical officer of Karolinska University Hospital, Johan Bratt, to report the whistleblowers to Swedish police, claiming that they violated the law and the patients' privacy when they went through the patients´ charts and submitted their appeals for investigation to KI and the Central Ethical Review Board. KI claimed that their report revealed the identities of patients, even though they had been careful to anonymize all the information. The police interrogated several of the whistleblowers and concluded that they had done no wrong, but the incident made it clear how low KI would sink in its desire to harass them.
"You can't appeal the findings. I don't know if I will ever get more research money. I'm totally dead."
In private, Grinnemo's colleagues supported him, but feared coming forward out of the fear of losing their jobs. Grinnemo himself was in a tough spot. "I knew it would be difficult for me to do research but I hoped my position as a surgeon was secure," he says. "But after the New York Times article, I realized even that position was not as safe as I had thought."
THE MEDIA CATCHES ON -- WITH A PRICE
On November 24, 2014, The New York Times published a front-page story about Paolo Macchiarini based on the whistleblowers' investigation, which had leaked to the press. Officials at KI suspected one or more of the whistleblowers of being the leakers, but the publicity forced the top brass to at least appear to act. The next day they asked Dr. Bengt Gerdin, a professor of surgery at Sweden's Uppsala University, to do an investigation of Dr. Macchiarini. It's hard not to conclude that, after months of stonewalling on an institutional investigation, the Times article compelled them to do something. But KI still did not take any of the pressure off of Grinnemo and his three fellow whistleblowers.
One by one, each was informed that he would receive a formal warning from Dr. Lockowandt, the head of the cardiothoracic clinic, alleging that they had violated patient privacy by reading medical records. The whistleblowers countered that they had informed consents. They also asked for a meeting with Lockowandt and KI's attorneys, to which they brought a union representative and someone from the Swedish version of the American Medical Association. The union representative informed KI's attorneys that the doctors were actually required by law to consult a patient's medical records when the patient's life is in danger. Not doing so would have been a crime. Karolinska backed off on the formal warnings (which would have been the last step before actual termination) after that. But they found other ways to retaliate.
One whistleblower, Oscar Simonson, had been offered a residency at Karolinska University Hospital, but that offer was withdrawn without explanation. Grinnemo had expected to receive an advisor position in cardiothoracic surgery, but that promotion also evaporated. In addition, the number of surgeries he was tapped to perform was reduced and he was relegated to doing the "less popular" standard heart surgeries that began late in the afternoon and evenings.
The grinding day-to-day pressure on the whistleblowers never let up. On December 19, 2014, Dr. Lockowandt informed all four that they had been on the verge of being fired, but that hospital attorneys changed their minds at the last minute. By then not only were their reputations in tatters, but they had invested an estimated 10,000 hours of labor investigating Macchiarini's misconduct, appealing to KI, and defending themselves against KI's harassment.
When interviewed for this article, Grinnemo said, "I have never had a single day of vacation from this situation. In addition to dealing with it, I've been doing surgery and taking care of patients. I've had trouble sleeping, and it has affected my family. I haven't been able to focus on my family, and I feel guilty toward my kids." Of all the whistleblowers, Grinnemo seems to have received the brunt of the backlash.
KI was finally pushed to further action by yet more negative coverage of the Macchiarini affair in the media. In January 2015, Swedish National Television aired an exposé covering the Macchiarini surgeries and the desperate plight of the patients. In response, the Swedish public demanded that KI make a course correction. On February 19, KI withdrew all of its threats of formal warnings to the whistleblowers.
As the press event began, KI called the heads of the whistleblowers' departments to tell them to make sure the four didn't attend.
However, progress was incremental. On April 16, KI's ethical committee, which had done its own investigation, acquitted Macchiarini of allegations of scientific misconduct. This is the same university ethical board that had reprimanded Grinnemo over his usage of data in the aortic valve grant application.
The whistleblowers maintain that throughout the summer of 2015, KI was still far more focused on covering up the Macchiarini affair than on getting to the bottom of it. On May 13, the professor from Uppsala submitted the results of his independent investigation, in which he concluded that seven out of seven published articles in which Macchiarini was the lead author entailed the fabrication of data.
KI ignored the report. In August 2015, KI's president announced that Macchiarini had been cleared of all charges of scientific misconduct and that, magically, ethical approvals existed for the patient from Iceland. Macchiarini got a reprimand for being "a little sloppy" in his published descriptions of his patients. Then KI, eager to placate the public and salvage its reputation, held a press conference to announce the presumed innocence of its star surgeon.
As the press event began, KI called the heads of the whistleblowers' departments to tell them to make sure the four didn't attend, according to Grinnemo.
"They seemed to think we would come crashing in to the press conference and make a scene. It's ridiculous, but that's what they thought," says Grinnemo.
Around this time, KI asked that the whistleblowers compile and forward all of their correspondence with the independent investigator on the grounds that they were suspected of manipulating his investigation. The accusation went nowhere; the whistleblowers had barely spoken with him.
Then came a request from KI's IT department for the whistleblowers to compile and submit all of their emails for the preceding year. They were simply told that "an anonymous person" had made the request.
Throughout 2015, KI continued to go after the whistleblowers aggressively. That August, they were so discouraged that they felt they would never obtain any additional grants from the Swedish Research Council or any other funding organizations, and that their academic careers were over. To add insult to injury, a Swedish newspaper published an article defending Macchiarini and concluding that he was not guilty of violating the Helsinki Declaration, a statute put into effect after World War II protecting all humans from unauthorized medical experimentation.
THE TIDE TURNS, BUT REDEMPTION IS ELUSIVE
Then in November, they received a request from a Swedish filmmaker to be interviewed about the Macchiarini affair. Not knowing what angle the film was expected to take, they each put in hours in front of the camera. They wouldn't know the results of their interviews until January 2016, when the three-part documentary, "The Experiments," aired on Swedish television. The film documented the tortuous death of a Russian woman and the suffering of other patients who had received Macchiarini's implants.
That same month, a devastating article on Paolo Macchiarini was published in the American magazine Vanity Fair. Titled "The Celebrity Surgeon Who Used Love, Money and the Pope to Scam an NBC News Producer," the article revealed Macchiarini as an even more prolific fabulist and liar than anyone had remotely suspected. Not only did he fabricate data for multiple scientific papers, he had also lied about everything from his alleged medical training and celebrity connections to his personal relationship status.
Ironically, the woman who ultimately dismantled Macchiarini was Benita Alexander, a former producer for NBC News who was at one point engaged to marry him in a lavish ceremony that Macchiarini promised would be officiated by Pope Francis. Except that he didn't know the Pope, and he was already married to one woman and living with another.
Her story of heartbreak infuriated the public. The full list of people who had believed Macchiarini's almost countless fabrications may never be known—a tribute to his considerable personal charisma. But after the "The Experiments" and the Vanity Fair article, the public had had enough of Paolo Macchiarini. They demanded that KI's president step down and that Macchiarini be fired.

TV producer Benita Alexander appeared as a guest on Dr. Oz's show on February 14th, 2018 to discuss Dr. Macchiarini's deception. "He railroaded my life," she said.
In February 2016, there was a cascade of resignations and firings at KI. First, president Anders Hamsten stepped down. Then several top KI officials, including the General Secretary of the Nobel Assembly, the Dean of Research, and an advisor to KI's president, were either fired or stepped down. On March 3, several members of the board were replaced. The whistleblowers received an award for coming forward by an organization called Transparency International, but instead of heaving a sigh of relief, they only felt a continued sense of foreboding.
"We all felt very vulnerable because we knew that KI would retaliate in some way," says Grinnemo. A fellow whistleblower, Dr. Corbascio, gave an interview on a prime time news program saying that KI was a corrupt institution and should apologize to the patients' families and even pay them for their suffering. After that, both he and another colleague came under intensified scrutiny at work. They say that their supervisors, who were deeply involved in collaborations with Macchiarini, watched everything they did, apparently looking for a reason to fire them.
Grinnemo and Simonson both left KI to work for Uppsala University. But the lasting effects of the scandal followed them there. They still couldn't obtain any grants for new research, and other scientists at KI and elsewhere were unwilling to collaborate with them for fear of their own work being "tainted" by association.
On March 23, 2016, Paolo Macchiarini was finally sacked by KI. Still, the whistleblowers couldn't claim victory.
"Our aim," says Grinnemo, "was not to get him sacked but to stop the grafts, and we knew he would continue to do them in other countries. The clinical trial aiming to recruit 100 or so patients hadn't been halted. We tried to warn the Russian authorities and the EU grant office, and wanted them to stop the grant to Macchiarini. There was no response, so at that time we didn't know if the clinical trial would go forward."
Still, there was reason to hope. News of Macchiarini's scientific fraud, not to mention his personal debacle with Benita Alexander, had made its way around scientific circles in Germany and Britain, where a new investigation began.
Eventually, the entire board at Karolinska was replaced. Under its new president, the institute issued a decree this past summer finding the now thoroughly disgraced Macchiarini guilty of scientific misconduct, and concluding that six of his research papers should be retracted.
But in a cruelly ironic twist, KI took the whistleblowers' own investigation and turned it against them. KI's report found Grinnemo also guilty of scientific misconduct for apparently falling short in the care of the Icelandic patient, even though his role in the case had been minimal. It was like a punch in the gut, because the judgment cast Grinnemo as equally blameworthy to Macchiarini. It also failed to recognize that he had long ago not only withdrawn his name from the offending paper, but lobbied for years to have it retracted.
"This sends the message that whistleblowers in research will be punished. That's a serious problem."
The KI report also established the new category of "blameworthy" to describe two of the whistleblowers for their roles as co-authors in some of the papers. The whistleblowers did not receive a chance to respond to the new accusations before a decision was made to publicly reprimand them.
That decision can't be appealed.
Simonson told Science Magazine, "This sends the message that whistleblowers in research will be punished. That's a serious problem."
These days, Macchiarini is lying low but still publishing his supposed stem cell research, most recently on baboons. A paper published in March of this year in the Journal of Biomedical Materials lists his affiliation as Kazan Federal University in Russia, but in April 2017, the university fired him. He's rumored to be living in Italy and couldn't be reached for this article. He was investigated for criminal activity in Sweden and the case was closed without charges, but Grinnemo says that another prosecutor is now considering whether to bring charges against him for "aggravated manslaughter."
At KI, only Karin Dahlman Wright, who was the Institute's acting president during several months of these events, responded to a request for comment, but she claimed a near-total unawareness of the whistleblowers' narrative. Other officials there declined to be interviewed.
KI's clinical trial that was aiming to recruit new patients for biologically engineered tracheas is no longer happening. The European Commission posted on their research portal that the trial ended on March 31, 2017, stating: "Grant Agreement terminated."
As for Grinnemo, Simonson, Corbascio and Fux, they are still fighting for their careers. Grinnemo is currently suing KI for a chance to defend himself against its accusations of scientific misconduct. He's also claiming damages for lost grant funding, thousands of hours spent defending himself, and harm to his reputation. Whether he will prevail in court remains to be seen.
"KI did a very good job of destroying our careers," says Simonson. "They didn't do anything else well, but they did a very thorough job of that."
Researchers claimed they built a breakthrough superconductor. Social media shot it down almost instantly.
In July, South Korean scientists posted a paper finding they had achieved superconductivity - a claim that was debunked within days.
Harsh Mathur was a graduate physics student at Yale University in late 1989 when faculty announced they had failed to replicate claims made by scientists at the University of Utah and the University of Wolverhampton in England.
Such work is routine. Replicating or attempting to replicate the contraptions, calculations and conclusions crafted by colleagues is foundational to the scientific method. But in this instance, Yale’s findings were reported globally.
“I had a ringside view, and it was crazy,” recalls Mathur, now a professor of physics at Case Western Reserve University in Ohio.
Yale’s findings drew so much attention because initial experiments by Stanley Pons of Utah and Martin Fleischmann of Wolverhampton led to a startling claim: They were able to fuse atoms at room temperature – a scientific El Dorado known as “cold fusion.”
Nuclear fusion powers the stars in the universe. However, star cores must be at least 23.4 million degrees Fahrenheit and under extraordinary pressure to achieve fusion. Pons and Fleischmann claimed they had created an almost limitless source of power achievable at any temperature.
Like fusion, superconductivity can only be achieved in mostly impractical circumstances.
But about six months after they made their startling announcement, the pair’s findings were discredited by researchers at Yale and the California Institute of Technology. It was one of the first instances of a major scientific debunking covered by mass media.
Some scholars say the media attention for cold fusion stemmed partly from a dazzling announcement made three years prior in 1986: Scientists had created the first “superconductor” – material that could transmit electrical current with little or no resistance. It drew global headlines – and whetted the public’s appetite for announcements of scientific breakthroughs that could cause economic transformations.
But like fusion, superconductivity can only be achieved in mostly impractical circumstances: It must operate either at temperatures of at least negative 100 degrees Fahrenheit, or under pressures of around 150,000 pounds per square inch. Superconductivity that functions in closer to a normal environment would cut energy costs dramatically while also opening infinite possibilities for computing, space travel and other applications.
In July, a group of South Korean scientists posted material claiming they had created an iron crystalline substance called LK-99 that could achieve superconductivity at slightly above room temperature and at ambient pressure. The group partners with the Quantum Energy Research Centre, a privately-held enterprise in Seoul, and their claims drew global headlines.
Their work was also debunked. But in the age of internet and social media, the process was compressed from half-a-year into days. And it did not require researchers at world-class universities.
One of the most compelling critiques came from Derrick VanGennep. Although he works in finance, he holds a Ph.D. in physics and held a postdoctoral position at Harvard. The South Korean researchers had posted a video of a nugget of LK-99 in what they claimed was the throes of the Meissner effect – an expulsion of the substance’s magnetic field that would cause it to levitate above a magnet. Unless Hollywood magic is involved, only superconducting material can hover in this manner.
That claim made VanGennep skeptical, particularly since LK-99’s levitation appeared unenthusiastic at best. In fact, a corner of the material still adhered to the magnet near its center. He thought the video demonstrated ferromagnetism – two magnets repulsing one another. He mixed powdered graphite with super glue, stuck iron filings to its surface and mimicked the behavior of LK-99 in his own video, which was posted alongside the researchers’ video.
VanGennep believes the boldness of the South Korean claim was what led to him and others in the scientific community questioning it so quickly.
“The swift replication attempts stemmed from the combination of the extreme claim, the fact that the synthesis for this material is very straightforward and fast, and the amount of attention that this story was getting on social media,” he says.
But practicing scientists were suspicious of the data as well. Michael Norman, director of the Argonne Quantum Institute at the Argonne National Laboratory just outside of Chicago, had doubts immediately.
Will this saga hurt or even affect the careers of the South Korean researchers? Possibly not, if the previous fusion example is any indication.
“It wasn’t a very polished paper,” Norman says of the Korean scientists’ work. That opinion was reinforced, he adds, when it turned out the paper had been posted online by one of the researchers prior to seeking publication in a peer-reviewed journal. Although Norman and Mathur say that is routine with scientific research these days, Norman notes it was posted by one of the junior researchers over the doubts of two more senior scientists on the project.
Norman also raises doubts about the data reported. Among other issues, he observes that the samples created by the South Korean researchers contained traces of copper sulfide that could inadvertently amplify findings of conductivity.
The lack of the Meissner effect also caught Mathur’s attention. “Ferromagnets tend to be unstable when they levitate,” he says, adding that the video “just made me feel unconvinced. And it made me feel like they hadn't made a very good case for themselves.”
Will this saga hurt or even affect the careers of the South Korean researchers? Possibly not, if the previous fusion example is any indication. Despite being debunked, cold fusion claimants Pons and Fleischmann didn’t disappear. They moved their research to automaker Toyota’s IMRA laboratory in France, which along with the Japanese government spent tens of millions of dollars on their work before finally pulling the plug in 1998.
Fusion has since been created in laboratories, but being unable to reproduce the density of a star’s core would require excruciatingly high temperatures to achieve – about 160 million degrees Fahrenheit. A recently released Government Accountability Office report concludes practical fusion likely remains at least decades away.
However, like Pons and Fleischman, the South Korean researchers are not going anywhere. They claim that LK-99’s Meissner effect is being obscured by the fact the substance is both ferromagnetic and diamagnetic. They have filed for a patent in their country. But for now, those claims remain chimerical.
In the meantime, the consensus as to when a room temperature superconductor will be achieved is mixed. VenGennep – who studied the issue during his graduate and postgraduate work – puts the chance of creating such a superconductor by 2050 at perhaps 50-50. Mathur believes it could happen sooner, but adds that research on the topic has been going on for nearly a century, and that it has seen many plateaus.
“There's always this possibility that there's going to be something out there that we're going to discover unexpectedly,” Norman notes. The only certainty in this age of social media is that it will be put through the rigors of replication instantly.
Scientists implant brain cells to counter Parkinson's disease
In a recent research trial, patients with Parkinson's disease reported that their symptoms had improved after stem cells were implanted into their brains. Martin Taylor, far right, was diagnosed at age 32.
Martin Taylor was only 32 when he was diagnosed with Parkinson's, a disease that causes tremors, stiff muscles and slow physical movement - symptoms that steadily get worse as time goes on.
“It's horrible having Parkinson's,” says Taylor, a data analyst, now 41. “It limits my ability to be the dad and husband that I want to be in many cruel and debilitating ways.”
Today, more than 10 million people worldwide live with Parkinson's. Most are diagnosed when they're considerably older than Taylor, after age 60. Although recent research has called into question certain aspects of the disease’s origins, Parkinson’s eventually kills the nerve cells in the brain that produce dopamine, a signaling chemical that carries messages around the body to control movement. Many patients have lost 60 to 80 percent of these cells by the time they are diagnosed.
For years, there's been little improvement in the standard treatment. Patients are typically given the drug levodopa, a chemical that's absorbed by the brain’s nerve cells, or neurons, and converted into dopamine. This drug addresses the symptoms but has no impact on the course of the disease as patients continue to lose dopamine producing neurons. Eventually, the treatment stops working effectively.
BlueRock Therapeutics, a cell therapy company based in Massachusetts, is taking a different approach by focusing on the use of stem cells, which can divide into and generate new specialized cells. The company makes the dopamine-producing cells that patients have lost and inserts these cells into patients' brains. “We have a disease with a high unmet need,” says Ahmed Enayetallah, the senior vice president and head of development at BlueRock. “We know [which] cells…are lost to the disease, and we can make them. So it really came together to use stem cells in Parkinson's.”
In a phase 1 research trial announced late last month, patients reported that their symptoms had improved after a year of treatment. Brain scans also showed an increased number of neurons generating dopamine in patients’ brains.
Increases in dopamine signals
The recent phase 1 trial focused on deploying BlueRock’s cell therapy, called bemdaneprocel, to treat 12 patients suffering from Parkinson’s. The team developed the new nerve cells and implanted them into specific locations on each side of the patient's brain through two small holes in the skull made by a neurosurgeon. “We implant cells into the places in the brain where we think they have the potential to reform the neural networks that are lost to Parkinson's disease,” Enayetallah says. The goal is to restore motor function to patients over the long-term.
Five patients were given a relatively low dose of cells while seven got higher doses. Specialized brain scans showed evidence that the transplanted cells had survived, increasing the overall number of dopamine producing cells. The team compared the baseline number of these cells before surgery to the levels one year later. “The scans tell us there is evidence of increased dopamine signals in the part of the brain affected by Parkinson's,” Enayetallah says. “Normally you’d expect the signal to go down in untreated Parkinson’s patients.”
"I think it has a real chance to reverse motor symptoms, essentially replacing a missing part," says Tilo Kunath, a professor of regenerative neurobiology at the University of Edinburgh.
The team also asked patients to use a specific type of home diary to log the times when symptoms were well controlled and when they prevented normal activity. After a year of treatment, patients taking the higher dose reported symptoms were under control for an average of 2.16 hours per day above their baselines. At the smaller dose, these improvements were significantly lower, 0.72 hours per day. The higher-dose patients reported a corresponding decrease in the amount of time when symptoms were uncontrolled, by an average of 1.91 hours, compared to 0.75 hours for the lower dose. The trial was safe, and patients tolerated the year of immunosuppression needed to make sure their bodies could handle the foreign cells.
Claire Bale, the associate director of research at Parkinson's U.K., sees the promise of BlueRock's approach, while noting the need for more research on a possible placebo effect. The trial participants knew they were getting the active treatment, and placebo effects are known to be a potential factor in Parkinson’s research. Even so, “The results indicate that this therapy produces improvements in symptoms for Parkinson's, which is very encouraging,” Bale says.
Tilo Kunath, a professor of regenerative neurobiology at the University of Edinburgh, also finds the results intriguing. “I think it's excellent,” he says. “I think it has a real chance to reverse motor symptoms, essentially replacing a missing part.” However, it could take time for this therapy to become widely available, Kunath says, and patients in the late stages of the disease may not benefit as much. “Data from cell transplantation with fetal tissue in the 1980s and 90s show that cells did not survive well and release dopamine in these [late-stage] patients.”
Searching for the right approach
There's a long history of using cell therapy as a treatment for Parkinson's. About four decades ago, scientists at the University of Lund in Sweden developed a method in which they transferred parts of fetal brain tissue to patients with Parkinson's so that their nerve cells would produce dopamine. Many benefited, and some were able to stop their medication. However, the use of fetal tissue was highly controversial at that time, and the tissues were difficult to obtain. Later trials in the U.S. showed that people benefited only if a significant amount of the tissue was used, and several patients experienced side effects. Eventually, the work lost momentum.
“Like many in the community, I'm aware of the long history of cell therapy,” says Taylor, the patient living with Parkinson's. “They've long had that cure over the horizon.”
In 2000, Lorenz Studer led a team at the Memorial Sloan Kettering Centre, in New York, to find the chemical signals needed to get stem cells to differentiate into cells that release dopamine. Back then, the team managed to make cells that produced some dopamine, but they led to only limited improvements in animals. About a decade later, in 2011, Studer and his team found the specific signals needed to guide embryonic cells to become the right kind of dopamine producing cells. Their experiments in mice, rats and monkeys showed that their implanted cells had a significant impact, restoring lost movement.
Studer then co-founded BlueRock Therapeutics in 2016. Forming the most effective stem cells has been one of the biggest challenges, says Enayetallah, the BlueRock VP. “It's taken a lot of effort and investment to manufacture and make the cells at the right scale under the right conditions.” The team is now using cells that were first isolated in 1998 at the University of Wisconsin, a major advantage because they’re available in a virtually unlimited supply.
Other efforts underway
In the past several years, University of Lund researchers have begun to collaborate with the University of Cambridge on a project to use embryonic stem cells, similar to BlueRock’s approach. They began clinical trials this year.
A company in Japan called Sumitomo is using a different strategy; instead of stem cells from embryos, they’re reprogramming adults' blood or skin cells into induced pluripotent stem cells - meaning they can turn into any cell type - and then directing them into dopamine producing neurons. Although Sumitomo started clinical trials earlier than BlueRock, they haven’t yet revealed any results.
“It's a rapidly evolving field,” says Emma Lane, a pharmacologist at the University of Cardiff who researches clinical interventions for Parkinson’s. “But BlueRock’s trial is the first full phase 1 trial to report such positive findings with stem cell based therapies.” The company’s upcoming phase 2 research will be critical to show how effectively the therapy can improve disease symptoms, she added.
The cure over the horizon
BlueRock will continue to look at data from patients in the phase 1 trial to monitor the treatment’s effects over a two-year period. Meanwhile, the team is planning the phase 2 trial with more participants, including a placebo group.
For patients with Parkinson’s like Martin Taylor, the therapy offers some hope, though Taylor recognizes that more research is needed.

BlueRock Therapeutics
“Like many in the community, I'm aware of the long history of cell therapy,” he says. “They've long had that cure over the horizon.” His expectations are somewhat guarded, he says, but, “it's certainly positive to see…movement in the field again.”
"If we can demonstrate what we’re seeing today in a more robust study, that would be great,” Enayetallah says. “At the end of the day, we want to address that unmet need in a field that's been waiting for a long time.”
Editor's note: The company featured in this piece, BlueRock Therapeutics, is a portfolio company of Leaps by Bayer, which is a sponsor of Leaps.org. BlueRock was acquired by Bayer Pharmaceuticals in 2019. Leaps by Bayer and other sponsors have never exerted influence over Leaps.org content or contributors.