A vaccine for ovarian cancer is now in development

The upcoming vaccine is changing the way we look at treating one of the country’s deadliest cancers.
Last week, researchers at the University of Oxford announced that they have received funding to create a brand new way of preventing ovarian cancer: A vaccine. The vaccine, known as OvarianVax, will teach the immune system to recognize and destroy mutated cells—one of the earliest indicators of ovarian cancer.
Understanding Ovarian Cancer
Despite advancements in medical research and treatment protocols over the last few decades, ovarian cancer still poses a significant threat to women’s health. In the United States alone, more than 12,0000 women die of ovarian cancer each year, and only about half of women diagnosed with ovarian cancer survive five or more years past diagnosis. Unlike cervical cancer, there is no routine screening for ovarian cancer, so it often goes undetected until it has reached advanced stages. Additionally, the primary symptoms of ovarian cancer—frequent urination, bloating, loss of appetite, and abdominal pain—can often be mistaken for other non-cancerous conditions, delaying treatment.
An American woman has roughly a one percent chance of developing ovarian cancer throughout her lifetime. However, these odds increase significantly if she has inherited mutations in the BRCA1 or BRCA2 genes. Women who carry these mutations face a 46% lifetime risk for ovarian and breast cancers.
An Unlikely Solution
To address this escalating health concern, the organization Cancer Research UK has invested £600,000 over the next three years in research aimed at creating a vaccine, which would destroy cancerous cells before they have a chance to develop any further.
Researchers at the University of Oxford are at the forefront of this initiative. With funding from Cancer Research UK, scientists will use tissue samples from the ovaries and fallopian tubes of patients currently battling ovarian cancer. Using these samples, University of Oxford scientists will create a vaccine to recognize certain proteins on the surface of ovarian cancer cells known as tumor-associated antigens. The vaccine will then train that person’s immune system to recognize the cancer markers and destroy them.
The next step
Once developed, the vaccine will first be tested in patients with the disease, to see if their ovarian tumors will shrink or disappear. Then, the vaccine will be tested in women with the BRCA1 or BRCA2 mutations as well as women in the general population without genetic mutations, to see whether the vaccine can prevent the cancer altogether.
While the vaccine still has “a long way to go,” according to Professor Ahmed Ahmed, Director of Oxford University’s ovarian cancer cell laboratory, he is “optimistic” about the results.
“We need better strategies to prevent ovarian cancer,” said Ahmed in a press release from the University of Oxford. “Currently, women with BRCA1/2 mutations are offered surgery which prevents cancer but robs them of the chance to have children afterward.
Teaching the immune system to recognize the very early signs of cancer is a tough challenge. But we now have highly sophisticated tools which give us real insights into how the immune system recognizes ovarian cancer. OvarianVax could offer the solution.”
When doctors couldn’t stop her daughter’s seizures, this mom earned a PhD and found a treatment herself.
Savannah Salazar (left) and her mother, Tracy Dixon-Salazaar, who earned a PhD in neurobiology in the quest for a treatment of her daughter's seizure disorder.
Twenty-eight years ago, Tracy Dixon-Salazaar woke to the sound of her daughter, two-year-old Savannah, in the midst of a medical emergency.
“I entered [Savannah’s room] to see her tiny little body jerking about violently in her bed,” Tracy said in an interview. “I thought she was choking.” When she and her husband frantically called 911, the paramedic told them it was likely that Savannah had had a seizure—a term neither Tracy nor her husband had ever heard before.
Over the next several years, Savannah’s seizures continued and worsened. By age five Savannah was having seizures dozens of times each day, and her parents noticed significant developmental delays. Savannah was unable to use the restroom and functioned more like a toddler than a five-year-old.
Doctors were mystified: Tracy and her husband had no family history of seizures, and there was no event—such as an injury or infection—that could have caused them. Doctors were also confused as to why Savannah’s seizures were happening so frequently despite trying different seizure medications.
Doctors eventually diagnosed Savannah with Lennox-Gaustaut Syndrome, or LGS, an epilepsy disorder with no cure and a poor prognosis. People with LGS are often resistant to several kinds of anti-seizure medications, and often suffer from developmental delays and behavioral problems. People with LGS also have a higher chance of injury as well as a higher chance of sudden unexpected death (SUDEP) due to the frequent seizures. In about 70 percent of cases, LGS has an identifiable cause such as a brain injury or genetic syndrome. In about 30 percent of cases, however, the cause is unknown.
Watching her daughter struggle through repeated seizures was devastating to Tracy and the rest of the family.
“This disease, it comes into your life. It’s uninvited. It’s unannounced and it takes over every aspect of your daily life,” said Tracy in an interview with Today.com. “Plus it’s attacking the thing that is most precious to you—your kid.”
Desperate to find some answers, Tracy began combing the medical literature for information about epilepsy and LGS. She enrolled in college courses to better understand the papers she was reading.
“Ironically, I thought I needed to go to college to take English classes to understand these papers—but soon learned it wasn’t English classes I needed, It was science,” Tracy said. When she took her first college science course, Tracy says, she “fell in love with the subject.”
Tracy was now a caregiver to Savannah, who continued to have hundreds of seizures a month, as well as a full-time student, studying late into the night and while her kids were at school, using classwork as “an outlet for the pain.”
“I couldn’t help my daughter,” Tracy said. “Studying was something I could do.”
Twelve years later, Tracy had earned a PhD in neurobiology.
After her post-doctoral training, Tracy started working at a lab that explored the genetics of epilepsy. Savannah’s doctors hadn’t found a genetic cause for her seizures, so Tracy decided to sequence her genome again to check for other abnormalities—and what she found was life-changing.
Tracy discovered that Savannah had a calcium channel mutation, meaning that too much calcium was passing through Savannah’s neural pathways, leading to seizures. The information made sense to Tracy: Anti-seizure medications often leech calcium from a person’s bones. When doctors had prescribed Savannah calcium supplements in the past to counteract these effects, her seizures had gotten worse every time she took the medication. Tracy took her discovery to Savannah’s doctor, who agreed to prescribe her a calcium blocker.
The change in Savannah was almost immediate.
Within two weeks, Savannah’s seizures had decreased by 95 percent. Once on a daily seven-drug regimen, she was soon weaned to just four, and then three. Amazingly, Tracy started to notice changes in Savannah’s personality and development, too.
“She just exploded in her personality and her talking and her walking and her potty training and oh my gosh she is just so sassy,” Tracy said in an interview.
Since starting the calcium blocker eleven years ago, Savannah has continued to make enormous strides. Though still unable to read or write, Savannah enjoys puzzles and social media. She’s “obsessed” with boys, says Tracy. And while Tracy suspects she’ll never be able to live independently, she and her daughter can now share more “normal” moments—something she never anticipated at the start of Savannah’s journey with LGS. While preparing for an event, Savannah helped Tracy get ready.
“We picked out a dress and it was the first time in our lives that we did something normal as a mother and a daughter,” she said. “It was pretty cool.”
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
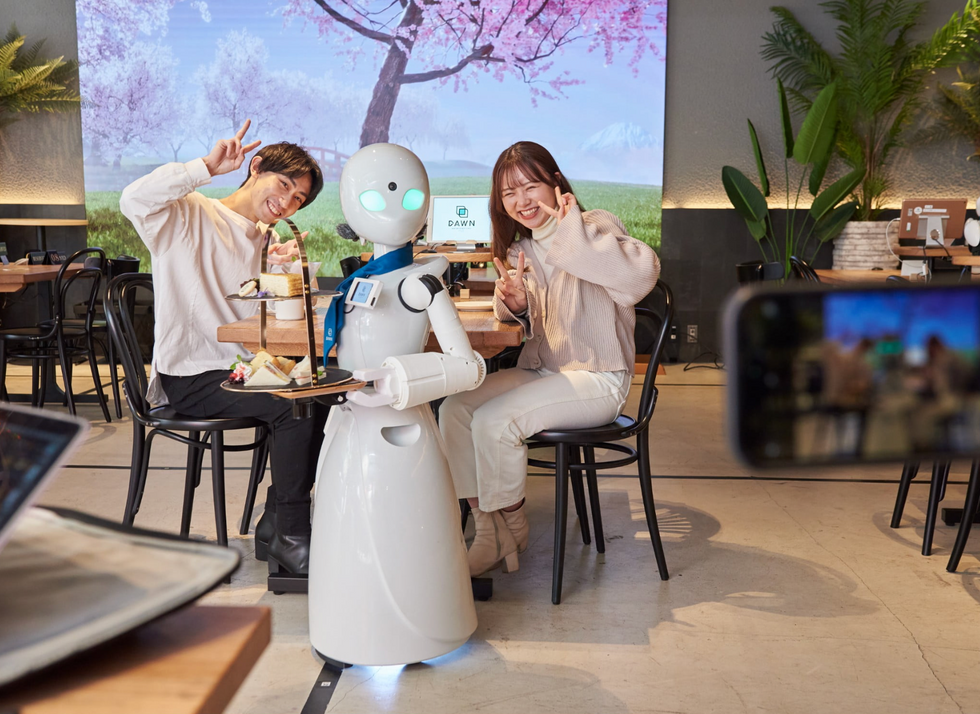
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
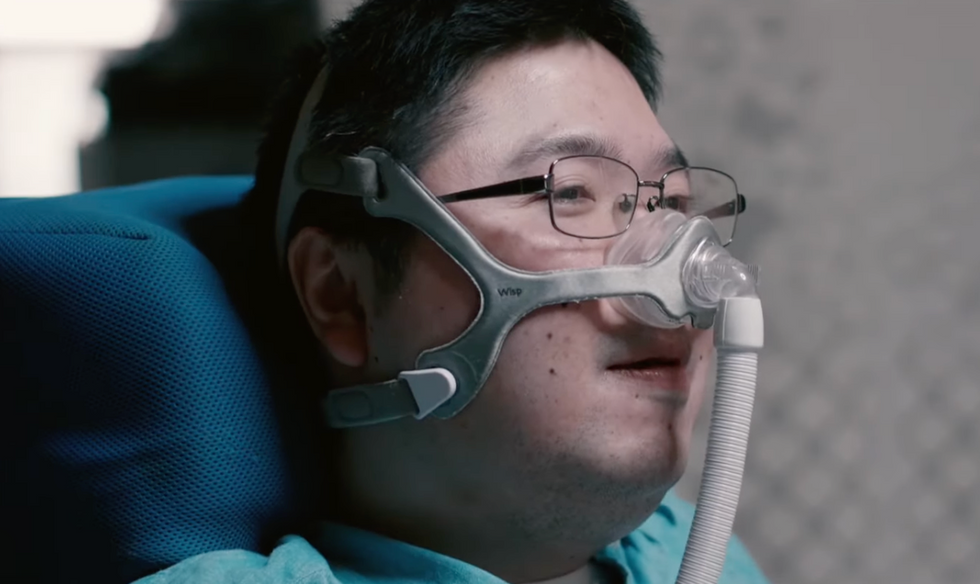
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."

