Opioid prescription policies may hurt those in chronic pain

Guidelines aim to prevent opioid-related deaths by making it more challenging to get prescriptions, but they can also block access for those who desperately need them.
Tinu Abayomi-Paul works as a writer and activist, plus one unwanted job: Trying to fill her opioid prescription. She says that some pharmacists laugh and tell her that no one needs the amount of pain medication that she is seeking. Another pharmacist near her home in Venus, Tex., refused to fill more than seven days of a 30-day prescription.
To get a new prescription—partially filled opioid prescriptions can’t be dispensed later—Abayomi-Paul needed to return to her doctor’s office. But without her medication, she was having too much pain to travel there, much less return to the pharmacy. She rationed out the pills over several weeks, an agonizing compromise that left her unable to work, interact with her children, sleep restfully, or leave the house. “Don’t I deserve to do more than survive?” she says.
Abayomi-Paul’s pain results from a degenerative spine disorder, chronic lymphocytic leukemia, and more than a dozen other diagnoses and disabilities. She is part of a growing group of people with chronic pain who have been negatively impacted by the fallout from efforts to prevent opioid overdose deaths.
Guidelines for dispensing these pills are complicated because many opioids, like codeine, oxycodone, and morphine, are prescribed legally for pain. Yet, deaths from opioids have increased rapidly since 1999 and become a national emergency. Many of them, such as heroin, are used illegally. The CDC identified three surges in opioid use: an increase in opioid prescriptions in the ‘90s, a surge of heroin around 2010, and an influx of fentanyl and other powerful synthetic opioids in 2013.
As overdose deaths grew, so did public calls to address them, prompting the CDC to change its prescription guidelines in 2016. The new guidelines suggested limiting medication for acute pain to a seven-day supply, capping daily doses of morphine, and other restrictions. Some statistics suggest that these policies have worked; from 2016 to 2019, prescriptions for opiates fell 44 percent. Physicians also started progressively lowering opioid doses for patients, a practice called tapering. A study tracking nearly 100,000 Medicare subscribers on opioids found that about 13 percent of patients were tapering in 2012, and that number increased to about 23 percent by 2017.
But some physicians may be too aggressive with this tapering strategy. About one in four people had doses reduced by more than 10 percent per week, a rate faster than the CDC recommends. The approach left people like Abayomi-Paul without the medication they needed. Every year, Abayomi-Paul says, her prescriptions are harder to fill. David Brushwood, a pharmacy professor who specializes in policy and outcomes at the University of Florida in Gainesville, says opioid dosing isn’t one-size-fits-all. “Patients need to be taken care of individually, not based on what some government agency says they need,” he says.
‘This is not survivable’
Health policy and disability rights attorney Erin Gilmer advocated for people with pain, using her own experience with chronic pain and a host of medical conditions as a guidepost. She launched an advocacy website, Healthcare as a Human Right, and shared her struggles on Twitter: “This pain is more than anything I've endured before and I've already been through too much. Yet because it's not simply identified no one believes it's as bad as it is. This is not survivable.”
When her pain dramatically worsened midway through 2021, Gilmer’s posts grew ominous: “I keep thinking it can't possibly get worse but somehow every day is worse than the last.”
The CDC revised its guidelines in 2022 after criticisms that people with chronic pain were being undertreated, enduring dangerous withdrawal symptoms, and suffering psychological distress. (Long-term opioid use can cause physical dependency, an adaptive reaction that is different than the compulsive misuse associated with a substance use disorder.) It was too late for Gilmer. On July 7, 2021, the 38-year-old died by suicide.
Last August, an Ohio district court ruling set forth a new requirement for Walgreens, Walmart, and CVS pharmacists in two counties. These pharmacists must now document opioid prescriptions that are turned down, even for customers who have no previous purchases at that pharmacy, and they’re required to share this information with other locations in the same chain. None of the three pharmacies responded to an interview request from Leaps.org.
In a practice called red flagging, pharmacists may label a prescription suspicious for a variety of reasons, such as if a pharmacist observes an unusually high dose, a long distance from the patient’s home to the pharmacy, or cash payment. Pharmacists may question patients or prescribers to resolve red flags but, regardless of the explanation, they’re free to refuse to fill a prescription.
As the risk of litigation has grown, so has finger-pointing, says Seth Whitelaw, a compliance consultant at Whitelaw Compliance Group in West Chester, PA, who advises drug, medical device, and biotech companies. Drugmakers accused in National Prescription Opioid Litigation (NPOL), a complex set of thousands of cases on opioid epidemic deaths, which includes the Ohio district case, have argued that they shouldn’t be responsible for the large supply of opiates and overdose deaths. Yet, prosecutors alleged that these pharmaceutical companies hid addiction and overdose risks when labeling opioids, while distributors and pharmacists failed to identify suspicious orders or scripts.
Patients and pharmacists fear red flags
The requirements that pharmacists document prescriptions they refuse to fill so far only apply to two counties in Ohio. But Brushwood fears they will spread because of this precedent, and because there’s no way for pharmacists to predict what new legislation is on the way. “There is no definition of a red flag, there are no lists of red flags. There is no instruction on what to do when a red flag is detected. There’s no guidance on how to document red flags. It is a standardless responsibility,” Brushwood says. This adds trepidation for pharmacists—and more hoops to jump through for patients.
“I went into the doctor one day here and she said, ‘I'm going to stop prescribing opioids to all my patients effective immediately,” Nicolson says.
“We now have about a dozen studies that show that actually ripping somebody off their medication increases their risk of overdose and suicide by three to five times, destabilizes their health and mental health, often requires some hospitalization or emergency care, and can cause heart attacks,” says Kate Nicolson, founder of the National Pain Advocacy Center based in Boulder, Colorado. “It can kill people.” Nicolson was in pain for decades due to a surgical injury to the nerves leading to her spinal cord before surgeries fixed the problem.
Another issue is that primary care offices may view opioid use as a reason to turn down new patients. In a 2021 study, secret shoppers called primary care clinics in nine states, identifying themselves as long-term opioid users. When callers said their opioids were discontinued because their former physician retired, as opposed to an unspecified reason, they were more likely to be offered an appointment. Even so, more than 40 percent were refused an appointment. The study authors say their findings suggest that some physicians may try to avoid treating people who use opioids.
Abayomi-Paul says red flagging has changed how she fills prescriptions. “Once I go to one place, I try to [continue] going to that same place because of the amount of records that I have and making sure my medications don’t conflict,” Abayomi-Paul says.
Nicolson moved to Colorado from Washington D.C. in 2015, before the CDC issued its 2016 guidelines. When the guidelines came out, she found the change to be shockingly abrupt. “I went into the doctor one day here and she said, ‘I'm going to stop prescribing opioids to all my patients effective immediately.’” Since then, she’s spoken with dozens of patients who have been red-flagged or simply haven’t been able to access pain medication.
Despite her expertise, Nicolson isn’t positive she could successfully fill an opioid prescription today even if she needed one. At this point, she’s not sure exactly what various pharmacies would view as a red flag. And she’s not confident that these red flags even work. “You can have very legitimate reasons for being 50 miles away or having to go to multiple pharmacies, given that there are drug shortages now, as well as someone refusing to fill [a prescription.] It doesn't mean that you’re necessarily ‘drug seeking.’”
While there’s no easy solution. Whitelaw says clarifying the role of pharmacists and physicians in patient access to opioids could help people get the medication they need. He is seeking policy changes that focus on the needs of people in pain more than the number of prescriptions filled. He also advocates standardizing the definition of red flags and procedures for resolving them. Still, there will never be a single policy that can be applied to all people, explains Brushwood, the University of Florida professor. “You have to make a decision about each individual prescription.”
Probiotic bacteria can be engineered to fight antibiotic-resistant superbugs by releasing chemicals that kill them.
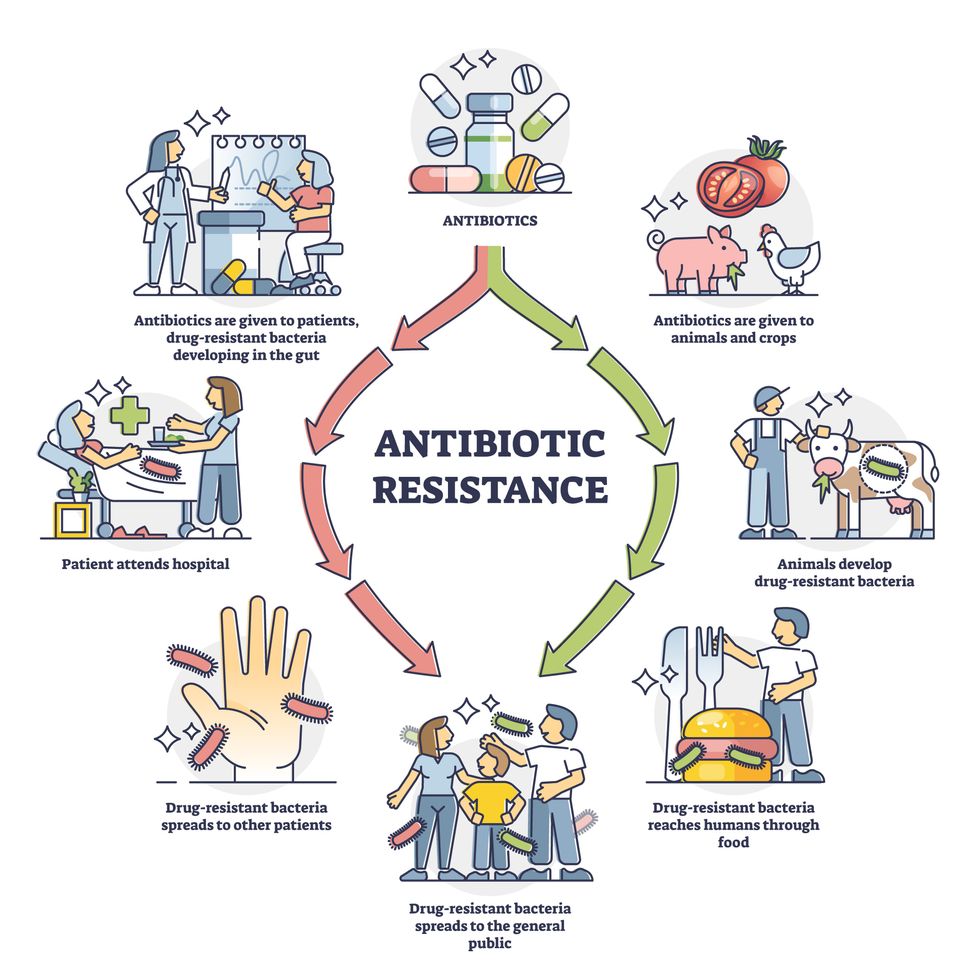
In 1945, almost two decades after Alexander Fleming discovered penicillin, he warned that as antibiotics use grows, they may lose their efficiency. He was prescient—the first case of penicillin resistance was reported two years later. Back then, not many people paid attention to Fleming’s warning. After all, the “golden era” of the antibiotics age had just began. By the 1950s, three new antibiotics derived from soil bacteria — streptomycin, chloramphenicol, and tetracycline — could cure infectious diseases like tuberculosis, cholera, meningitis and typhoid fever, among others.
Today, these antibiotics and many of their successors developed through the 1980s are gradually losing their effectiveness. The extensive overuse and misuse of antibiotics led to the rise of drug resistance. The livestock sector buys around 80 percent of all antibiotics sold in the U.S. every year. Farmers feed cows and chickens low doses of antibiotics to prevent infections and fatten up the animals, which eventually causes resistant bacterial strains to evolve. If manure from cattle is used on fields, the soil and vegetables can get contaminated with antibiotic-resistant bacteria. Another major factor is doctors overprescribing antibiotics to humans, particularly in low-income countries. Between 2000 to 2018, the global rates of human antibiotic consumption shot up by 46 percent.
In recent years, researchers have been exploring a promising avenue: the use of synthetic biology to engineer new bacteria that may work better than antibiotics. The need continues to grow, as a Lancet study linked antibiotic resistance to over 1.27 million deaths worldwide in 2019, surpassing HIV/AIDS and malaria. The western sub-Saharan Africa region had the highest death rate (27.3 people per 100,000).
Researchers warn that if nothing changes, by 2050, antibiotic resistance could kill 10 million people annually.
To make it worse, our remedy pipelines are drying up. Out of the 18 biggest pharmaceutical companies, 15 abandoned antibiotic development by 2013. According to the AMR Action Fund, venture capital has remained indifferent towards biotech start-ups developing new antibiotics. In 2019, at least two antibiotic start-ups filed for bankruptcy. As of December 2020, there were 43 new antibiotics in clinical development. But because they are based on previously known molecules, scientists say they are inadequate for treating multidrug-resistant bacteria. Researchers warn that if nothing changes, by 2050, antibiotic resistance could kill 10 million people annually.
The rise of synthetic biology
To circumvent this dire future, scientists have been working on alternative solutions using synthetic biology tools, meaning genetically modifying good bacteria to fight the bad ones.
From the time life evolved on earth around 3.8 billion years ago, bacteria have engaged in biological warfare. They constantly strategize new methods to combat each other by synthesizing toxic proteins that kill competition.
For example, Escherichia coli produces bacteriocins or toxins to kill other strains of E.coli that attempt to colonize the same habitat. Microbes like E.coli (which are not all pathogenic) are also naturally present in the human microbiome. The human microbiome harbors up to 100 trillion symbiotic microbial cells. The majority of them are beneficial organisms residing in the gut at different compositions.
The chemicals that these “good bacteria” produce do not pose any health risks to us, but can be toxic to other bacteria, particularly to human pathogens. For the last three decades, scientists have been manipulating bacteria’s biological warfare tactics to our collective advantage.
In the late 1990s, researchers drew inspiration from electrical and computing engineering principles that involve constructing digital circuits to control devices. In certain ways, every cell in living organisms works like a tiny computer. The cell receives messages in the form of biochemical molecules that cling on to its surface. Those messages get processed within the cells through a series of complex molecular interactions.
Synthetic biologists can harness these living cells’ information processing skills and use them to construct genetic circuits that perform specific instructions—for example, secrete a toxin that kills pathogenic bacteria. “Any synthetic genetic circuit is merely a piece of information that hangs around in the bacteria’s cytoplasm,” explains José Rubén Morones-Ramírez, a professor at the Autonomous University of Nuevo León, Mexico. Then the ribosome, which synthesizes proteins in the cell, processes that new information, making the compounds scientists want bacteria to make. “The genetic circuit remains separated from the living cell’s DNA,” Morones-Ramírez explains. When the engineered bacteria replicates, the genetic circuit doesn’t become part of its genome.
Highly intelligent by bacterial standards, some multidrug resistant V. cholerae strains can also “collaborate” with other intestinal bacterial species to gain advantage and take hold of the gut.
In 2000, Boston-based researchers constructed an E.coli with a genetic switch that toggled between turning genes on and off two. Later, they built some safety checks into their bacteria. “To prevent unintentional or deleterious consequences, in 2009, we built a safety switch in the engineered bacteria’s genetic circuit that gets triggered after it gets exposed to a pathogen," says James Collins, a professor of biological engineering at MIT and faculty member at Harvard University’s Wyss Institute. “After getting rid of the pathogen, the engineered bacteria is designed to switch off and leave the patient's body.”
Overuse and misuse of antibiotics causes resistant strains to evolve
Adobe Stock
Seek and destroy
As the field of synthetic biology developed, scientists began using engineered bacteria to tackle superbugs. They first focused on Vibrio cholerae, which in the 19th and 20th century caused cholera pandemics in India, China, the Middle East, Europe, and Americas. Like many other bacteria, V. cholerae communicate with each other via quorum sensing, a process in which the microorganisms release different signaling molecules, to convey messages to its brethren. Highly intelligent by bacterial standards, some multidrug resistant V. cholerae strains can also “collaborate” with other intestinal bacterial species to gain advantage and take hold of the gut. When untreated, cholera has a mortality rate of 25 to 50 percent and outbreaks frequently occur in developing countries, especially during floods and droughts.
Sometimes, however, V. cholerae makes mistakes. In 2008, researchers at Cornell University observed that when quorum sensing V. cholerae accidentally released high concentrations of a signaling molecule called CAI-1, it had a counterproductive effect—the pathogen couldn’t colonize the gut.
So the group, led by John March, professor of biological and environmental engineering, developed a novel strategy to combat V. cholerae. They genetically engineered E.coli to eavesdrop on V. cholerae communication networks and equipped it with the ability to release the CAI-1 molecules. That interfered with V. cholerae progress. Two years later, the Cornell team showed that V. cholerae-infected mice treated with engineered E.coli had a 92 percent survival rate.
These findings inspired researchers to sic the good bacteria present in foods like yogurt and kimchi onto the drug-resistant ones.
Three years later in 2011, Singapore-based scientists engineered E.coli to detect and destroy Pseudomonas aeruginosa, an often drug-resistant pathogen that causes pneumonia, urinary tract infections, and sepsis. Once the genetically engineered E.coli found its target through its quorum sensing molecules, it then released a peptide, that could eradicate 99 percent of P. aeruginosa cells in a test-tube experiment. The team outlined their work in a Molecular Systems Biology study.
“At the time, we knew that we were entering new, uncharted territory,” says lead author Matthew Chang, an associate professor and synthetic biologist at the National University of Singapore and lead author of the study. “To date, we are still in the process of trying to understand how long these microbes stay in our bodies and how they might continue to evolve.”
More teams followed the same path. In a 2013 study, MIT researchers also genetically engineered E.coli to detect P. aeruginosa via the pathogen’s quorum-sensing molecules. It then destroyed the pathogen by secreting a lab-made toxin.
Probiotics that fight
A year later in 2014, a Nature study found that the abundance of Ruminococcus obeum, a probiotic bacteria naturally occurring in the human microbiome, interrupts and reduces V.cholerae’s colonization— by detecting the pathogen’s quorum sensing molecules. The natural accumulation of R. obeum in Bangladeshi adults helped them recover from cholera despite living in an area with frequent outbreaks.
The findings from 2008 to 2014 inspired Collins and his team to delve into how good bacteria present in foods like yogurt and kimchi can attack drug-resistant bacteria. In 2018, Collins and his team developed the engineered probiotic strategy. They tweaked a bacteria commonly found in yogurt called Lactococcus lactis to treat cholera.
Engineered bacteria can be trained to target pathogens when they are at their most vulnerable metabolic stage in the human gut. --José Rubén Morones-Ramírez.
More scientists followed with more experiments. So far, researchers have engineered various probiotic organisms to fight pathogenic bacteria like Staphylococcus aureus (leading cause of skin, tissue, bone, joint and blood infections) and Clostridium perfringens (which causes watery diarrhea) in test-tube and animal experiments. In 2020, Russian scientists engineered a probiotic called Pichia pastoris to produce an enzyme called lysostaphin that eradicated S. aureus in vitro. Another 2020 study from China used an engineered probiotic bacteria Lactobacilli casei as a vaccine to prevent C. perfringens infection in rabbits.
In a study last year, Ramírez’s group at the Autonomous University of Nuevo León, engineered E. coli to detect quorum-sensing molecules from Methicillin-resistant Staphylococcus aureus or MRSA, a notorious superbug. The E. coli then releases a bacteriocin that kills MRSA. “An antibiotic is just a molecule that is not intelligent,” says Ramírez. “On the other hand, engineered bacteria can be trained to target pathogens when they are at their most vulnerable metabolic stage in the human gut.”
Collins and Timothy Lu, an associate professor of biological engineering at MIT, found that engineered E. coli can help treat other conditions—such as phenylketonuria, a rare metabolic disorder, that causes the build-up of an amino acid phenylalanine. Their start-up Synlogic aims to commercialize the technology, and has completed a phase 2 clinical trial.
Circumventing the challenges
The bacteria-engineering technique is not without pitfalls. One major challenge is that beneficial gut bacteria produce their own quorum-sensing molecules that can be similar to those that pathogens secrete. If an engineered bacteria’s biosensor is not specific enough, it will be ineffective.
Another concern is whether engineered bacteria might mutate after entering the gut. “As with any technology, there are risks where bad actors could have the capability to engineer a microbe to act quite nastily,” says Collins of MIT. But Collins and Ramírez both insist that the chances of the engineered bacteria mutating on its own are virtually non-existent. “It is extremely unlikely for the engineered bacteria to mutate,” Ramírez says. “Coaxing a living cell to do anything on command is immensely challenging. Usually, the greater risk is that the engineered bacteria entirely lose its functionality.”
However, the biggest challenge is bringing the curative bacteria to consumers. Pharmaceutical companies aren’t interested in antibiotics or their alternatives because it’s less profitable than developing new medicines for non-infectious diseases. Unlike the more chronic conditions like diabetes or cancer that require long-term medications, infectious diseases are usually treated much quicker. Running clinical trials are expensive and antibiotic-alternatives aren’t lucrative enough.
“Unfortunately, new medications for antibiotic resistant infections have been pushed to the bottom of the field,” says Lu of MIT. “It's not because the technology does not work. This is more of a market issue. Because clinical trials cost hundreds of millions of dollars, the only solution is that governments will need to fund them.” Lu stresses that societies must lobby to change how the modern healthcare industry works. “The whole world needs better treatments for antibiotic resistance.”
Meet Dr. Renee Wegrzyn, the first Director of President Biden's new health agency, ARPA-H
Today's podcast guest, Dr. Renee Wegrzyn, directs ARPA-H, a new agency formed last year to spearhead health innovations. Time will tell if ARPA-H will produce advances on the level of its fellow agency, DARPA.
In today’s podcast episode, I talk with Renee Wegrzyn, appointed by President Biden as the first director of a health agency created last year, the Advanced Research Projects Agency for Health, or ARPA-H. It’s inspired by DARPA, the agency that develops innovations for the Defense department and has been credited with hatching world-changing technologies such as ARPANET, which became the internet.
Time will tell if ARPA-H will lead to similar achievements in the realm of health. That’s what President Biden and Congress expect in return for funding ARPA-H at 2.5 billion dollars over three years.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
How will the agency figure out which projects to take on, especially with so many patient advocates for different diseases demanding moonshot funding for rapid progress?
I talked with Dr. Wegrzyn about the opportunities and challenges, what lessons ARPA-H is borrowing from Operation Warp Speed, how she decided on the first ARPA-H project that was announced recently, why a separate agency was needed instead of reforming HHS and the National Institutes of Health to be better at innovation, and how ARPA-H will make progress on disease prevention in addition to treatments for cancer, Alzheimer’s and diabetes, among many other health priorities.
Dr. Wegrzyn’s resume leaves no doubt of her suitability for this role. She was a program manager at DARPA where she focused on applying gene editing and synthetic biology to the goal of improving biosecurity. For her work there, she received the Superior Public Service Medal and, in case that wasn’t enough ARPA experience, she also worked at another ARPA that leads advanced projects in intelligence, called I-ARPA. Before that, she ran technical teams in the private sector working on gene therapies and disease diagnostics, among other areas. She has been a vice president of business development at Gingko Bioworks and headed innovation at Concentric by Gingko. Her training and education includes a PhD and undergraduate degree in applied biology from the Georgia Institute of Technology and she did her postdoc as an Alexander von Humboldt Fellow in Heidelberg, Germany.
Dr. Wegrzyn told me that she’s “in the hot seat.” The pressure is on for ARPA-H especially after the need and potential for health innovation was spot lit by the pandemic and the unprecedented speed of vaccine development. We'll soon find out if ARPA-H can produce gamechangers in health that are equivalent to DARPA’s creation of the internet.
Show links:
ARPA-H - https://arpa-h.gov/
Dr. Wegrzyn profile - https://arpa-h.gov/people/renee-wegrzyn/
Dr. Wegrzyn Twitter - https://twitter.com/rwegrzyn?lang=en
President Biden Announces Dr. Wegrzyn's appointment - https://www.whitehouse.gov/briefing-room/statement...
Leaps.org coverage of ARPA-H - https://leaps.org/arpa/
ARPA-H program for joints to heal themselves - https://arpa-h.gov/news/nitro/ -
ARPA-H virtual talent search - https://arpa-h.gov/news/aco-talent-search/

Dr. Renee Wegrzyn was appointed director of ARPA-H last October.