Breakthrough therapies are breaking patients' banks. Key changes could improve access, experts say.
Single-treatment therapies are revolutionizing medicine. But insurers and patients wonder whether they can afford treatment and, if they can, whether the high costs are worthwhile.
CSL Behring’s new gene therapy for hemophilia, Hemgenix, costs $3.5 million for one treatment, but helps the body create substances that allow blood to clot. It appears to be a cure, eliminating the need for other treatments for many years at least.
Likewise, Novartis’s Kymriah mobilizes the body’s immune system to fight B-cell lymphoma, but at a cost $475,000. For patients who respond, it seems to offer years of life without the cancer progressing.
These single-treatment therapies are at the forefront of a new, bold era of medicine. Unfortunately, they also come with new, bold prices that leave insurers and patients wondering whether they can afford treatment and, if they can, whether the high costs are worthwhile.
“Most pharmaceutical leaders are there to improve and save people’s lives,” says Jeremy Levin, chairman and CEO of Ovid Therapeutics, and immediate past chairman of the Biotechnology Innovation Organization. If the therapeutics they develop are too expensive for payers to authorize, patients aren’t helped.
“The right to receive care and the right of pharmaceuticals developers to profit should never be at odds,” Levin stresses. And yet, sometimes they are.
Leigh Turner, executive director of the bioethics program, University of California, Irvine, notes this same tension between drug developers that are “seeking to maximize profits by charging as much as the market will bear for cell and gene therapy products and other medical interventions, and payers trying to control costs while also attempting to provide access to medical products with promising safety and efficacy profiles.”
Why Payers Balk
Health insurers can become skittish around extremely high prices, yet these therapies often accompany significant overall savings. For perspective, the estimated annual treatment cost for hemophilia exceeds $300,000. With Hemgenix, payers would break even after about 12 years.
But, in 12 years, will the patient still have that insurer? Therein lies the rub. U.S. payers, are used to a “pay-as-you-go” model, in which the lifetime costs of therapies typically are shared by multiple payers over many years, as patients change jobs. Single treatment therapeutics eliminate that cost-sharing ability.
"As long as formularies are based on profits to middlemen…Americans’ healthcare costs will continue to skyrocket,” says Patricia Goldsmith, the CEO of CancerCare.
“There is a phenomenally complex, bureaucratic reimbursement system that has grown, layer upon layer, during several decades,” Levin says. As medicine has innovated, payment systems haven’t kept up.
Therefore, biopharma companies begin working with insurance companies and their pharmacy benefit managers (PBMs), which act on an insurer’s behalf to decide which drugs to cover and by how much, early in the drug approval process. Their goal is to make sophisticated new drugs available while still earning a return on their investment.
New Payment Models
Pay-for-performance is one increasingly popular strategy, Turner says. “These models typically link payments to evidence generation and clinically significant outcomes.”
A biotech company called bluebird bio, for example, offers value-based pricing for Zynteglo, a $2.8 million possible cure for the rare blood disorder known as beta thalassaemia. It generally eliminates patients’ need for blood transfusions. The company is so sure it works that it will refund 80 percent of the cost of the therapy if patients need blood transfusions related to that condition within five years of being treated with Zynteglo.
In his February 2023 State of the Union speech, President Biden proposed three pilot programs to reduce drug costs. One of them, the Cell and Gene Therapy Access Model calls on the federal Centers for Medicare & Medicaid Services to establish outcomes-based agreements with manufacturers for certain cell and gene therapies.
A mortgage-style payment system is another, albeit rare, approach. Amortized payments spread the cost of treatments over decades, and let people change employers without losing their healthcare benefits.
Only about 14 percent of all drugs that enter clinical trials are approved by the FDA. Pharma companies, therefore, have an exigent need to earn a profit.
The new payment models that are being discussed aren’t solutions to high prices, says Bill Kramer, senior advisor for health policy at Purchaser Business Group on Health (PBGH), a nonprofit that seeks to lower health care costs. He points out that innovative pricing models, although well-intended, may distract from the real problem of high prices. They are attempts to “soften the blow. The best thing would be to charge a reasonable price to begin with,” he says.
Instead, he proposes making better use of research on cost and clinical effectiveness. The Institute for Clinical and Economic Review (ICER) conducts such research in the U.S., determining whether the benefits of specific drugs justify their proposed prices. ICER is an independent non-profit research institute. Its reports typically assess the degrees of improvement new therapies offer and suggest prices that would reflect that. “Publicizing that data is very important,” Kramer says. “Their results aren’t used to the extent they could and should be.” Pharmaceutical companies tend to price their therapies higher than ICER’s recommendations.
Drug Development Costs Soar
Drug developers have long pointed to the onerous costs of drug development as a reason for high prices.
A 2020 study found the average cost to bring a drug to market exceeded $1.1 billion, while other studies have estimated overall costs as high as $2.6 billion. The development timeframe is about 10 years. That’s because modern therapeutics target precise mechanisms to create better outcomes, but also have high failure rates. Only about 14 percent of all drugs that enter clinical trials are approved by the FDA. Pharma companies, therefore, have an exigent need to earn a profit.
Skewed Incentives Increase Costs
Pricing isn’t solely at the discretion of pharma companies, though. “What patients end up paying has much more to do with their PBMs than the actual price of the drug,” Patricia Goldsmith, CEO, CancerCare, says. Transparency is vital.
PBMs control patients’ access to therapies at three levels, through price negotiations, pricing tiers and pharmacy management.
When negotiating with drug manufacturers, Goldsmith says, “PBMs exchange a preferred spot on a formulary (the insurer’s or healthcare provider’s list of acceptable drugs) for cash-base rebates.” Unfortunately, 25 percent of the time, those rebates are not passed to insurers, according to the PBGH report.
Then, PBMs use pricing tiers to steer patients and physicians to certain drugs. For example, Kramer says, “Sometimes PBMs put a high-cost brand name drug in a preferred tier and a lower-cost competitor in a less preferred, higher-cost tier.” As the PBGH report elaborates, “(PBMs) are incentivized to include the highest-priced drugs…since both manufacturing rebates, as well as the administrative fees they charge…are calculated as a percentage of the drug’s price.
Finally, by steering patients to certain pharmacies, PBMs coordinate patients’ access to treatments, control patients’ out-of-pocket costs and receive management fees from the pharmacies.
Therefore, Goldsmith says, “As long as formularies are based on profits to middlemen…Americans’ healthcare costs will continue to skyrocket.”
Transparency into drug pricing will help curb costs, as will new payment strategies. What will make the most impact, however, may well be the development of a new reimbursement system designed to handle dramatic, breakthrough drugs. As Kramer says, “We need a better system to identify drugs that offer dramatic improvements in clinical care.”
Breakthrough drones deliver breast milk in rural Uruguay
A drone hovers over Tacuarembó hospital in Uruguay, carrying its precious cargo: breast milk intended for children in remote parts of the country.
Until three months ago, nurse Leopoldina Castelli used to send bottles of breast milk to nourish babies in the remote areas of Tacuarembó, in northern Uruguay, by way of ambulances or military trucks. That is, if the vehicles were available and the roads were passable, which wasn’t always the case. Now, five days per week, she stands by a runway at the hospital, located in Tacuarembó’s capital, watching a drone take off and disappear from view, carrying the milk to clinics that serve the babies’ families.
The drones can fly as far as 62 miles. Long distances and rough roads are no obstacles. The babies, whose mothers struggle to produce sufficient milk and cannot afford formula, now receive ample supplies for healthy growth. “Today we provided nourishment to a significantly larger number of children, and this is something that deeply moves me,” Castelli says.
About two decades ago, the Tacuarembó hospital established its own milk bank, supported by donations from mothers across Tacuarembó. Over the years, the bank has provided milk to infants immediately after birth. It's helped drive a “significant and sustained” decrease in infant mortality, says the hospital director, Ciro Ferreira.
But these children need breast milk throughout their first six months, if not longer, to prevent malnutrition and other illnesses that are prevalent in rural Tacuarembó. Ground transport isn't quick or reliable enough to meet this goal. It can take several hours, during which the milk may spoil due to a lack of refrigeration.
The battery-powered drones have been the difference-maker. The project to develop them, financed by the UNICEF Innovation Fund, is the first of its kind in Latin America. To Castelli, it's nothing short of a revolution. Tacuarembó Hospital, along with three rural clinics in the most impoverished part of Uruguay, are its leaders.
"This marks the first occasion when the public health system has been directly impacted [by our technology]," says Sebastián Macías, the CEO and co-founder of Cielum, an engineer at the University Republic, which collaborated on the technology with a Uruguayan company called Cielum and a Swiss company, Rigitech.
The drone can achieve a top speed of up to 68 miles per hour, is capable of flying in light rain, and can withstand winds of up to 30 miles per hour at a maximum altitude of 120 meters.
"We have succeeded in embracing the mothers from rural areas who were previously slipping through the cracks of the system," says Ferreira, the hospital director. He envisions an expansion of the service so it can improve health for children in other rural areas.

Nurses load the drone for breast milk delivery.
Sebastián Macías - Cielum
The star aircraft
The drone, which costs approximately $70,000, was specifically designed for the transportation of biological materials. Constructed from carbon fiber, it's three meters wide, two meters long and weighs 42 pounds when fully loaded. Additionally, it is equipped with a ballistic parachute to ensure a safe descent in case the technology fails in midair. Furthermore, it can achieve a top speed of 68 miles per hour, fly in light rain, and withstand winds of 30 miles per hour at a height of 120 meters.
Inside, the drones feature three refrigerated compartments that maintain a stable temperature and adhere to the United Nations’ standards for transporting perishable products. These compartments accommodate four gallons or 6.5 pounds of cargo. According to Macías, that's more than sufficient to carry a week’s worth of milk for one infant on just two flights, or 3.3 pounds of blood samples collected in a rural clinic.
“From an energy perspective, it serves as an efficient mode of transportation and helps reduce the carbon emissions associated with using an ambulance,” said Macías. Plus, the ambulance can remain available in the town.
Macías, who has led software development for the drone, and three other technicians have been trained to operate it. They ensure that the drone stays on course, monitor weather conditions and implement emergency changes when needed. The software displays the in-flight positions of the drones in relation to other aircraft. All agricultural planes in the region receive notification about the drone's flight path, departure and arrival times, and current location.
The future: doubling the drone's reach
Forty-five days after its inaugural flight, the drone is now making five flights per week. It serves two routes: 34 miles to Curtina and 31 miles to Tambores. The drone reaches Curtina in 50 minutes while ambulances take double that time, partly due to the subpar road conditions. Pueblo Ansina, located 40 miles from the state capital, will soon be introduced as the third destination.
Overall, the drone’s schedule is expected to become much busier, with plans to accomplish 20 weekly flights by the end of October and over 30 in 2024. Given the drone’s speed, Macías is contemplating using it to transport cancer medications as well.
“When it comes to using drones to save lives, for us, the sky is not the limit," says Ciro Ferreira, Tacuarembó hospital director.
In future trips to clinics in San Gregorio de Polanco and Caraguatá, the drone will be pushed to the limit. At these locations, a battery change will be necessary, but it's worth it. The route will cover up to 10 rural Tacuarembó clinics plus one hospital outside Tacuarembó, in Rivera, close to the border with Brazil. Currently, because of a shortage of ambulances, the delivery of pasteurized breast milk to Rivera only occurs every 15 days.
“The expansion to Rivera will include 100,000 more inhabitants, doubling the healthcare reach,” said Ferreira, the director of the Tacuarembó Hospital. In itself, Ferreira's hospital serves the medical needs of 500,000 people as one of the largest in Uruguay's interior.
Alejandro Del Estal, an aeronautical engineer at Rigitech, traveled from Europe to Tacuarembó to oversee the construction of the vertiports – the defined areas that can support drones’ take-off and landing – and the first flights. He pointed out that once the flight network between hospitals and rural polyclinics is complete in Uruguay, it will rank among the five most extensive drone routes in the world for any activity, including healthcare and commercial uses.
Cielum is already working on the long-term sustainability of the project. The aim is to have more drones operating in other rural regions in the western and northern parts of the country. The company has received inquiries from Argentina and Colombia, but, as Macías pointed out, they are exercising caution when making commitments. Expansion will depend on the development of each country’s regulations for airspace use.
For Ferreira, the advantages in Uruguay are evident: "This approach enables us to bridge the geographical gap, enhance healthcare accessibility, and reduce the time required for diagnosing and treating rural inhabitants, all without the necessity of them traveling to the hospital,” he says. "When it comes to using drones to save lives, for us, the sky is not the limit."
After spaceflight record, NASA looks to protect astronauts on even longer trips
NASA astronaut Frank Rubio floats by the International Space Station’s “window to the world.” Yesterday, he returned from the longest single spaceflight by a U.S. astronaut on record - over one year. Exploring deep space will require even longer missions.
At T-minus six seconds, the main engines of the Atlantis Space Shuttle ignited, rattling its capsule “like a skyscraper in an earthquake,” according to astronaut Tom Jones, describing the 1988 launch. As the rocket lifted off and accelerated to three times the force of Earth's gravity, “It felt as if two of my friends were standing on my chest and wouldn’t get off.” But when Atlantis reached orbit, the main engines cut off, and the astronauts were suddenly weightless.
Since 1961, NASA has sent hundreds of astronauts into space while working to making their voyages safer and smoother. Yet, challenges remain. Weightlessness may look amusing when watched from Earth, but it has myriad effects on cognition, movement and other functions. When missions to space stretch to six months or longer, microgravity can impact astronauts’ health and performance, making it more difficult to operate their spacecraft.
Yesterday, NASA astronaut Frank Rubio returned to Earth after over one year, the longest single spaceflight for a U.S. astronaut. But this is just the start; longer and more complex missions into deep space loom ahead, from returning to the moon in 2025 to eventually sending humans to Mars. To ensure that these missions succeed, NASA is increasing efforts to study the biological effects and prevent harm.
The dangers of microgravity are real
A NASA report published in 2016 details a long list of incidents and near-misses caused – at least partly – by space-induced changes in astronauts’ vision and coordination. These issues make it harder to move with precision and to judge distance and velocity.
According to the report, in 1997, a resupply ship collided with the Mir space station, possibly because a crew member bumped into the commander during the final docking maneuver. This mishap caused significant damage to the space station.
Returns to Earth suffered from problems, too. The same report notes that touchdown speeds during the first 100 space shuttle landings were “outside acceptable limits. The fastest landing on record – 224 knots (258 miles) per hour – was linked to the commander’s momentary spatial disorientation.” Earlier, each of the six Apollo crews that landed on the moon had difficulty recognizing moon landmarks and estimating distances. For example, Apollo 15 landed in an unplanned area, ultimately straddling the rim of a five-foot deep crater on the moon, harming one of its engines.
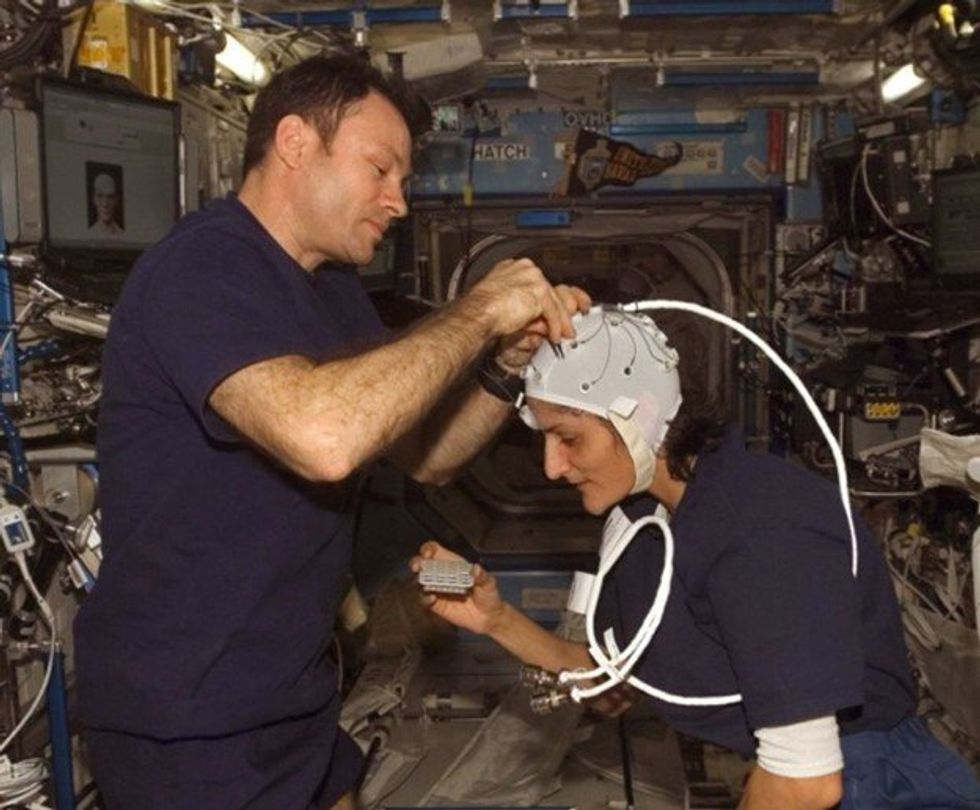
Spaceflight causes unique stresses on astronauts’ brains and central nervous systems. NASA is working to reduce these harmful effects.
NASA
Space messes up your brain
In space, astronauts face the challenges of microgravity, ionizing radiation, social isolation, high workloads, altered circadian rhythms, monotony, confined living quarters and a high-risk environment. Among these issues, microgravity is one of the most consequential in terms of physiological changes. It changes the brain’s structure and its functioning, which can hurt astronauts’ performance.
The brain shifts upwards within the skull, displacing the cerebrospinal fluid, which reduces the brain’s cushioning. Essentially, the brain becomes crowded inside the skull like a pair of too-tight shoes.
That’s partly because of how being in space alters blood flow. On Earth, gravity pulls our blood and other internal fluids toward our feet, but our circulatory valves ensure that the fluids are evenly distributed throughout the body. In space, there’s not enough gravity to pull the fluids down, and they shift up, says Rachael D. Seidler, a physiologist specializing in spaceflight at the University of Florida and principal investigator on many space-related studies. The head swells and legs appear thinner, causing what astronauts call “puffy face chicken legs.”
“The brain changes at the structural and functional level,” says Steven Jillings, equilibrium and aerospace researcher at the University of Antwerp in Belgium. “The brain shifts upwards within the skull,” displacing the cerebrospinal fluid, which reduces the brain’s cushioning. Essentially, the brain becomes crowded inside the skull like a pair of too-tight shoes. Some of the displaced cerebrospinal fluid goes into cavities within the brain, called ventricles, enlarging them. “The remaining fluids pool near the chest and heart,” explains Jillings. After 12 consecutive months in space, one astronaut had a ventricle that was 25 percent larger than before the mission.
Some changes reverse themselves while others persist for a while. An example of a longer-lasting problem is spaceflight-induced neuro-ocular syndrome, which results in near-sightedness and pressure inside the skull. A study of approximately 300 astronauts shows near-sightedness affects about 60 percent of astronauts after long missions on the International Space Station (ISS) and more than 25 percent after spaceflights of only a few weeks.
Another long-term change could be the decreased ability of cerebrospinal fluid to clear waste products from the brain, Seidler says. That’s because compressing the brain also compresses its waste-removing glymphatic pathways, resulting in inflammation, vulnerability to injuries and worsening its overall health.
The effects of long space missions were best demonstrated on astronaut twins Scott and Mark Kelly. This NASA Twins Study showed multiple, perhaps permanent, changes in Scott after his 340-day mission aboard the ISS, compared to Mark, who remained on Earth. The differences included declines in Scott’s speed, accuracy and cognitive abilities that persisted longer than six months after returning to Earth in March 2016.
By the end of 2020, Scott’s cognitive abilities improved, but structural and physiological changes to his eyes still remained, he said in a BBC interview.
“It seems clear that the upward shift of the brain and compression of the surrounding tissues with ventricular expansion might not be a good thing,” Seidler says. “But, at this point, the long-term consequences to brain health and human performance are not really known.”

NASA astronaut Kate Rubins conducts a session for the Neuromapping investigation.
NASA
Staying sharp in space
To investigate how prolonged space travel affects the brain, NASA launched a new initiative called the Complement of Integrated Protocols for Human Exploration Research (CIPHER). “CIPHER investigates how long-duration spaceflight affects both brain structure and function,” says neurobehavioral scientist Mathias Basner at the University of Pennsylvania, a principal investigator for several NASA studies. “Through it, we can find out how the brain adapts to the spaceflight environment and how certain brain regions (behave) differently after – relative to before – the mission.”
To do this, he says, “Astronauts will perform NASA’s cognition test battery before, during and after six- to 12-month missions, and will also perform the same test battery in an MRI scanner before and after the mission. We have to make sure we better understand the functional consequences of spaceflight on the human brain before we can send humans safely to the moon and, especially, to Mars.”
As we go deeper into space, astronauts cognitive and physical functions will be even more important. “A trip to Mars will take about one year…and will introduce long communication delays,” Seidler says. “If you are on that mission and have a problem, it may take eight to 10 minutes for your message to reach mission control, and another eight to 10 minutes for the response to get back to you.” In an emergency situation, that may be too late for the response to matter.
“On a mission to Mars, astronauts will be exposed to stressors for unprecedented amounts of time,” Basner says. To counter them, NASA is considering the continuous use of artificial gravity during the journey, and Seidler is studying whether artificial gravity can reduce the harmful effects of microgravity. Some scientists are looking at precision brain stimulation as a way to improve memory and reduce anxiety due to prolonged exposure to radiation in space.
Other scientists are exploring how to protect neural stem cells (which create brain cells) from radiation damage, developing drugs to repair damaged brain cells and protect cells from radiation.
To boldly go where no astronauts have gone before, they must have optimal reflexes, vision and decision-making. In the era of deep space exploration, the brain—without a doubt—is the final frontier.
Additionally, NASA is scrutinizing each aspect of the mission, including astronaut exercise, nutrition and intellectual engagement. “We need to give astronauts meaningful work. We need to stimulate their sensory, cognitive and other systems appropriately,” Basner says, especially given their extreme confinement and isolation. The scientific experiments performed on the ISS – like studying how microgravity affects the ability of tissue to regenerate is a good example.
“We need to keep them engaged socially, too,” he continues. The ISS crew, for example, regularly broadcasts from space and answers prerecorded questions from students on Earth, and can engage with social media in real time. And, despite tight quarters, NASA is ensuring the crew capsule and living quarters on the moon or Mars include private space, which is critical for good mental health.
Exploring deep space builds on a foundation that began when astronauts first left the planet. With each mission, scientists learn more about spaceflight effects on astronauts’ bodies. NASA will be using these lessons to succeed with its plans to build science stations on the moon and, eventually, Mars.
“Through internally and externally led research, investigations implemented in space and in spaceflight simulations on Earth, we are striving to reduce the likelihood and potential impacts of neurostructural changes in future, extended spaceflight,” summarizes NASA scientist Alexandra Whitmire. To boldly go where no astronauts have gone before, they must have optimal reflexes, vision and decision-making. In the era of deep space exploration, the brain—without a doubt—is the final frontier.

