After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.

Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.
New Device Can Detect Peanut Allergens on a Plate in 30 Seconds
Peanuts on a plate can be deadly for those with severe allergies, but an Israeli startup company wants to alleviate that fear.
People with life-threatening allergies live in constant fear of coming into contact with deadly allergens. Researchers estimate that about 32 million Americans have food allergies, with the most severe being milk, egg, peanut, tree nuts, wheat, soy, fish, and shellfish.
"It is important to understand that just several years ago, this would not have been possible."
Every three minutes, a food allergy reaction sends someone to the emergency room, and 200,000 people in the U.S. require emergency medical care each year for allergic reactions, according to Food Allergy Research and Education.
But what if there was a way you could easily detect if something you were about to eat contains any harmful allergens? Thanks to Israeli scientists, this will soon be the case — at least for peanuts. The team has been working to develop a handheld device called Allerguard, which analyzes the vapors in your meal and can detect allergens in 30 seconds.
Leapsmag spoke with the founder and CTO of Allerguard, Guy Ayal, about the groundbreaking technology, how it works, and when it will be available to purchase.
What prompted you to create this device? Do you have a personal connection with severe food allergies?
Guy Ayal: My eldest daughter's best friend suffers from a severe food allergy, and I experienced first-hand the effect it has on the person and their immediate surroundings. Most notable for me was the effect on the quality of life – the experience of living in constant fear. Everything we do at Allerguard is basically to alleviate some of that fear.
How exactly does the device work?
The device is built on two main pillars. The first is the nano-chemical stage, in which we developed specially attuned nanoparticles that selectively adhere only to the specific molecules that we are looking for. Those molecules, once bound to the nanoparticles, induce a change in their electrical behavior, which is measured and analyzed by the second main pillar -- highly advanced machine learning algorithms, which can surmise which molecules were collected, and thus whether or not peanuts (or in the future, other allergens) were detected.
It is important to understand that just several years ago, this would not have been possible, because both the nano-chemistry, and especially the entire world of machine learning, big data, and what is commonly known as AI only started to exist in the '90s, and reached applicability for handheld devices only in the past few years.
Where are you at in the development process and when will the device be available to consumers?
We have concluded the proof of concept and proof of capability phase, when we demonstrated successful detection of the minimal known clinical amount that may cause the slightest effect in the most severely allergic person – less than 1 mg of peanut (actually it is 0.7 mg). Over the next 18 months will be productization, qualification, and validation of our device, which should be ready to market in the latter half of 2021. The sensor will be available in the U.S., and after a year in Europe and Canada.

The Allerguard was made possible through recent advances in machine learning, big data, and AI.
(Courtesy)
How much will it cost?
Our target price is about $200 for the device, with a disposable SenseCard that will run for at least a full day and cost about $1. That card is for a specific allergen and will work for multiple scans in a day, not just one time.
[At a later stage, the company will have sensors for other allergens like tree nuts, eggs, and milk, and they'll develop a multi-SenseCard that works for a few allergens at once.]
Are there any other devices on the market that do something similar to Allerguard?
No other devices are even close to supplying the level of service that we promise. All known methods for allergen detection rely on sampling of the food, which is a viable solution for homogenous foodstuffs, such as a factory testing their raw ingredients, but not for something as heterogenous as an actual dish – especially not for solid allergens such as peanuts, treenuts, or sesame.
If there is a single peanut in your plate, and you sample from anywhere on that plate which is not where that peanut is located, you will find that your sample is perfectly clean – because it is. But the dish is not. That dish is a death trap for an allergic person. Allerguard is the only suggested solution that could indeed detect that peanut, no matter where in that plate it is hiding.
Anything else readers should know?
Our first-generation product will be for peanuts only. You have to understand, we are still a start-up company, and if we don't concentrate our limited resources to one specific goal, we will not be able to achieve anything at all. Once we are ready to market our first device, the peanut detector, we will be able to start the R&D for the 2nd product, which will be for another allergen – most likely tree nuts and/or sesame, but that will probably be in debate until we actually start it.
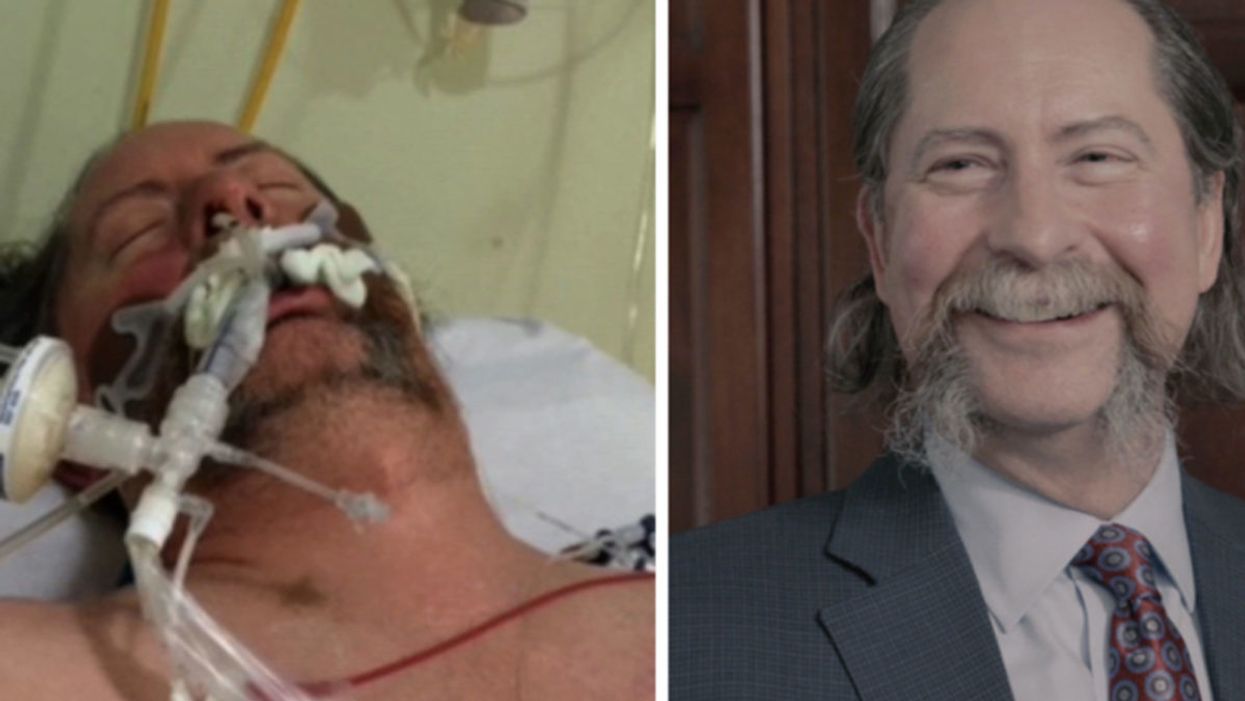
Dr. Robert Montgomery, the director of NYU Langone's Transplant Institute, is ironically the recipient of a heart transplant.
[Ed. Note: This is the fourth episode in our Moonshot series, which explores four cutting-edge scientific developments that stand to fundamentally transform our world.]
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.