How Leqembi became the biggest news in Alzheimer’s disease in 40 years, and what comes next

Betsy Groves, 73, with her granddaughter. Groves learned in 2021 that she has Alzheimer's disease. She hopes to take Leqembi, a drug approved by the FDA last week.
A few months ago, Betsy Groves traveled less than a mile from her home in Cambridge, Mass. to give a talk to a bunch of scientists. The scientists, who worked for the pharmaceutical companies Biogen and Eisai, wanted to know how she lived her life, how she thought about her future, and what it was like when a doctor’s appointment in 2021 gave her the worst possible news. Groves, 73, has Alzheimer’s disease. She caught it early, through a lumbar puncture that showed evidence of amyloid, an Alzheimer’s hallmark, in her cerebrospinal fluid. As a way of dealing with her diagnosis, she joined the Alzheimer’s Association’s National Early-Stage Advisory Board, which helped her shift into seeing her diagnosis as something she could use to help others.
After her talk, Groves stayed for lunch with the scientists, who were eager to put a face to their work. Biogen and Eisai were about to release the first drug to successfully combat Alzheimer’s in 40 years of experimental disaster. Their drug, which is known by the scientific name lecanemab and the marketing name Leqembi, was granted accelerated approval by the U.S. Food and Drug Administration last Friday, Jan. 6, after a study in 1,800 people showed that it reduced cognitive decline by 27 percent over 18 months.
It is no exaggeration to say that this result is a huge deal. The field of Alzheimer’s drug development has been absolutely littered with failures. Almost everything researchers have tried has tanked in clinical trials. “Most of the things that we've done have proven not to be effective, and it's not because we haven’t been taking a ton of shots at goal,” says Anton Porsteinsson, director of the University of Rochester Alzheimer's Disease Care, Research, and Education Program, who worked on the lecanemab trial. “I think it's fair to say you don't survive in this field unless you're an eternal optimist.”
As far back as 1984, a cure looked like it was within reach: Scientists discovered that the sticky plaques that develop in the brains of those who have Alzheimer’s are made up of a protein fragment called beta-amyloid. Buildup of beta-amyloid seemed to be sufficient to disrupt communication between, and eventually kill, memory cells. If that was true, then the cure should be straightforward: Stop the buildup of beta-amyloid; stop the Alzheimer’s disease.
It wasn’t so simple. Over the next 38 years, hundreds of drugs designed either to interfere with the production of abnormal amyloid or to clear it from the brain flamed out in trials. It got so bad that neuroscience drug divisions at major pharmaceutical companies (AstraZeneca, Pfizer, Bristol-Myers, GSK, Amgen) closed one by one, leaving the field to smaller, scrappier companies, like Cambridge-based Biogen and Tokyo-based Eisai. Some scientists began to dismiss the amyloid hypothesis altogether: If this protein fragment was so important to the disease, why didn’t ridding the brain of it do anything for patients? There was another abnormal protein that showed up in the brains of Alzheimer’s patients, called tau. Some researchers defected to the tau camp, or came to believe the proteins caused damage in combination.
The situation came to a head in 2021, when the FDA granted provisional approval to a drug called aducanumab, marketed as Aduhelm, against the advice of its own advisory council. The approval was based on proof that Aduhelm reduced beta-amyloid in the brain, even though one research trial showed it had no effect on people’s symptoms or daily life. Aduhelm could also cause serious side effects, like brain swelling and amyloid related imaging abnormalities (known as ARIA, these are basically micro-bleeds that appear on MRI scans). Without a clear benefit to memory loss that would make these risks worth it, Medicare refused to pay for Aduhelm among the general population. Two congressional committees launched an investigation into the drug’s approval, citing corporate greed, lapses in protocol, and an unjustifiably high price. (Aduhelm was also produced by the pharmaceutical company Biogen.)
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world.
So far, Leqembi is like Aduhelm in that it has been given accelerated approval only for its ability to remove amyloid from the brain. Both are monoclonal antibodies that direct the immune system to attack and clear dysfunctional beta-amyloid. The difference is that, while that’s all Aduhelm was ever shown to do, Leqembi’s makers have already asked the FDA to give it full approval – a decision that would increase the likelihood that Medicare will cover it – based on data that show it also improves Alzheimer’s sufferer’s lives. Leqembi targets a different type of amyloid, a soluble version called “protofibrils,” and that appears to change the effect. “It can give individuals and their families three, six months longer to be participating in daily life and living independently,” says Claire Sexton, PhD, senior director of scientific programs & outreach for the Alzheimer's Association. “These types of changes matter for individuals and for their families.”
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. It does not halt or reverse the disease, and people do not get better. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world. It has “a rather small effect,” wrote NIH Alzheimer’s researcher Madhav Thambisetty, MD, PhD, in an email to Leaps.org. “It is unclear how meaningful this difference will be to patients, and it is unlikely that this level of difference will be obvious to a patient (or their caregivers).” Another issue is cost: Leqembi will become available to patients later this month, but Eisai is setting the price at $26,500 per year, meaning that very few patients will be able to afford it unless Medicare chooses to reimburse them for it.
The same side effects that plagued Aduhelm are common in Leqembi treatment as well. In many patients, amyloid doesn’t just accumulate around neurons, it also forms deposits in the walls of blood vessels. Blood vessels that are shot through with amyloid are more brittle. If you infuse a drug that targets amyloid, brittle blood vessels in the brain can develop leakage that results in swelling or bleeds. Most of these come with no symptoms, and are only seen during testing, which is why they are called “imaging abnormalities.” But in situations where patients have multiple diseases or are prescribed incompatible drugs, they can be serious enough to cause death. The three deaths reported from Leqembi treatment (so far) are enough to make Thambisetty wonder “how well the drug may be tolerated in real world clinical practice where patients are likely to be sicker and have multiple other medical conditions in contrast to carefully selected patients in clinical trials.”
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval.
Still, there are reasons to be excited. A successful Alzheimer’s drug can pave the way for combination studies, in which patients try a known effective drug alongside newer, more experimental ones; or preventative studies, which take place years before symptoms occur. It also represents enormous strides in researchers’ understanding of the disease. For example, drug dosages have increased massively—in some cases quadrupling—from the early days of Alzheimer’s research. And patient selection for studies has changed drastically as well. Doctors now know that you’ve got to catch the disease early, through PET-scans or CSF tests for amyloid, if you want any chance of changing its course.
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval. His lab already uses blood tests for different types of amyloid, for different types of tau, and for measures of neuroinflammation, neural damage, and synaptic health, but commercially available versions from companies like C2N, Quest, and Fuji Rebio are likely to hit the market in the next couple of years. “[They are] going to transform the diagnosis of Alzheimer's disease,” Porsteinsson says. “If someone is experiencing memory problems, their physicians will be able to order a blood test that will tell us if this is the result of changes in your brain due to Alzheimer's disease. It will ultimately make it much easier to identify people at a very early stage of the disease, where they are most likely to benefit from treatment.”
Learn more about new blood tests to detect Alzheimer's
Early detection can help patients for more philosophical reasons as well. Betsy Groves credits finding her Alzheimer’s early with giving her the space to understand and process the changes that were happening to her before they got so bad that she couldn’t. She has been able to update her legal documents and, through her role on the Advisory Group, help the Alzheimer’s Association with developing its programs and support services for people in the early stages of the disease. She still drives, and because she and her husband love to travel, they are hoping to get out of grey, rainy Cambridge and off to Texas or Arizona this spring.
Because her Alzheimer’s disease involves amyloid deposits (a “substantial portion” do not, says Claire Sexton, which is an additional complication for research), and has not yet reached an advanced stage, Groves may be a good candidate to try Leqembi. She says she’d welcome the opportunity to take it. If she can get access, Groves hopes the drug will give her more days to be fully functioning with her husband, daughters, and three grandchildren. Mostly, she avoids thinking about what the latter stages of Alzheimer’s might be like, but she knows the time will come when it will be her reality. “So whatever lecanemab can do to extend my more productive ways of engaging with relationships in the world,” she says. “I'll take that in a minute.”
Researchers are looking to engineer chocolate with less oil, which could reduce some of its detriments to health.
Creamy milk with velvety texture. Dark with sprinkles of sea salt. Crunchy hazelnut-studded chunks. Chocolate is a treat that appeals to billions of people worldwide, no matter the age. And it’s not only the taste, but the feel of a chocolate morsel slowly melting in our mouths—the smoothness and slipperiness—that’s part of the overwhelming satisfaction. Why is it so enjoyable?
That’s what an interdisciplinary research team of chocolate lovers from the University of Leeds School of Food Science and Nutrition and School of Mechanical Engineering in the U.K. resolved to study in 2021. They wanted to know, “What is making chocolate that desirable?” says Siavash Soltanahmadi, one of the lead authors of a new study about chocolates hedonistic quality.
Besides addressing the researchers’ general curiosity, their answers might help chocolate manufacturers make the delicacy even more enjoyable and potentially healthier. After all, chocolate is a billion-dollar industry. Revenue from chocolate sales, whether milk or dark, is forecasted to grow 13 percent by 2027 in the U.K. In the U.S., chocolate and candy sales increased by 11 percent from 2020 to 2021, on track to reach $44.9 billion by 2026. Figuring out how chocolate affects the human palate could up the ante even more.
Building a 3D tongue
The team began by building a 3D tongue to analyze the physical process by which chocolate breaks down inside the mouth.
As part of the effort, reported earlier this year in the scientific journal ACS Applied Materials and Interfaces, the team studied a large variety of human tongues with the intention to build an “average” 3D model, says Soltanahmadi, a lubrication scientist. When it comes to edible substances, lubrication science looks at how food feels in the mouth and can help design foods that taste better and have more satisfying texture or health benefits.
There are variations in how people enjoy chocolate; some chew it while others “lick it” inside their mouths.
Tongue impressions from human participants studied using optical imaging helped the team build a tongue with key characteristics. “Our tongue is not a smooth muscle, it’s got some texture, it has got some roughness,” Soltanahmadi says. From those images, the team came up with a digital design of an average tongue and, using 3D printed molds, built a “mimic tongue.” They also added elastomers—such as silicone or polyurethane—to mimic the roughness, the texture and the mechanical properties of a real tongue. “Wettability" was another key component of the 3D tongue, Soltanahmadi says, referring to whether a surface mixes with water (hydrophilic) or, in the case of oil, resists it (hydrophobic).
Notably, the resulting artificial 3D-tongues looked nothing like the human version, but they were good mimics. The scientists also created “testing kits” that produced data on various physical parameters. One such parameter was viscosity, the measure of how gooey a food or liquid is — honey is more viscous compared to water, for example. Another was tribology, which defines how slippery something is — high fat yogurt is more slippery than low fat yogurt; milk can be more slippery than water. The researchers then mixed chocolate with artificial saliva and spread it on the 3D tongue to measure the tribology and the viscosity. From there they were able to study what happens inside the mouth when we eat chocolate.
The team focused on the stages of lubrication and the location of the fat in the chocolate, a process that has rarely been researched.

The artificial 3D-tongues look nothing like human tongues, but they function well enough to do the job.
Courtesy Anwesha Sarkar and University of Leeds
The oral processing of chocolate
We process food in our mouths in several stages, Soltanahmadi says. And there is variation in these stages depending on the type of food. So, the oral processing of a piece of meat would be different from, say, the processing of jelly or popcorn.
There are variations with chocolate, in particular; some people chew it while others use their tongues to explore it (within their mouths), Soltanahmadi explains. “Usually, from a consumer perspective, what we find is that if you have a luxury kind of a chocolate, then people tend to start with licking the chocolate rather than chewing it.” The researchers used a luxury brand of dark chocolate and focused on the process of licking rather than chewing.
As solid cocoa particles and fat are released, the emulsion envelops the tongue and coats the palette creating a smooth feeling of chocolate all over the mouth. That tactile sensation is part of the chocolate’s hedonistic appeal we crave.
Understanding the make-up of the chocolate was also an important step in the study. “Chocolate is a composite material. So, it has cocoa butter, which is oil, it has some particles in it, which is cocoa solid, and it has sugars," Soltanahmadi says. "Dark chocolate has less oil, for example, and less sugar in it, most of the time."
The researchers determined that the oral processing of chocolate begins as soon as it enters a person’s mouth; it starts melting upon exposure to one’s body temperature, even before the tongue starts moving, Soltanahmadi says. Then, lubrication begins. “[Saliva] mixes with the oily chocolate and it makes an emulsion." An emulsion is a fluid with a watery (or aqueous) phase and an oily phase. As chocolate breaks down in the mouth, that solid piece turns into a smooth emulsion with a fatty film. “The oil from the chocolate becomes droplets in a continuous aqueous phase,” says Soltanahmadi. In other words, as solid cocoa particles and fat are released, the emulsion envelops the tongue and coats the palette, creating a smooth feeling of chocolate all over the mouth. That tactile sensation is part of the chocolate’s hedonistic appeal we crave, says Soltanahmadi.
Finding the sweet spot
After determining how chocolate is orally processed, the research team wanted to find the exact sweet spot of the breakdown of solid cocoa particles and fat as they are released into the mouth. They determined that the epicurean pleasure comes only from the chocolate's outer layer of fat; the secondary fatty layers inside the chocolate don’t add to the sensation. It was this final discovery that helped the team determine that it might be possible to produce healthier chocolate that would contain less oil, says Soltanahmadi. And therefore, less fat.
Rongjia Tao, a physicist at Temple University in Philadelphia, thinks the Leeds study and the concept behind it is “very interesting.” Tao, himself, did a study in 2016 and found he could reduce fat in milk chocolate by 20 percent. He believes that the Leeds researchers’ discovery about the first layer of fat being more important for taste than the other layer can inform future chocolate manufacturing. “As a scientist I consider this significant and an important starting point,” he says.
Chocolate is rich in polyphenols, naturally occurring compounds also found in fruits and vegetables, such as grapes, apples and berries. Research found that plant polyphenols can protect against cancer, diabetes and osteoporosis as well as cardiovascular ad neurodegenerative diseases.
Not everyone thinks it’s a good idea, such as chef Michael Antonorsi, founder and owner of Chuao Chocolatier, one of the leading chocolate makers in the U.S. First, he says, “cacao fat is definitely a good fat.” Second, he’s not thrilled that science is trying to interfere with nature. “Every time we've tried to intervene and change nature, we get things out of balance,” says Antonorsi. “There’s a reason cacao is botanically known as food of the gods. The botanical name is the Theobroma cacao: Theobroma in ancient Greek, Theo is God and Brahma is food. So it's a food of the gods,” Antonorsi explains. He’s doubtful that a chocolate made only with a top layer of fat will produce the same epicurean satisfaction. “You're not going to achieve the same sensation because that surface fat is going to dissipate and there is no fat from behind coming to take over,” he says.
Without layers of fat, Antonorsi fears the deeply satisfying experiential part of savoring chocolate will be lost. The University of Leeds team, however, thinks that it may be possible to make chocolate healthier - when consumed in limited amounts - without sacrificing its taste. They believe the concept of less fatty but no less slick chocolate will resonate with at least some chocolate-makers and consumers, too.
Chocolate already contains some healthful compounds. Its cocoa particles have “loads of health benefits,” says Soltanahmadi. Dark chocolate usually has more cocoa than milk chocolate. Some experts recommend that dark chocolate should contain at least 70 percent cocoa in order for it to offer some health benefit. Research has shown that the cocoa in chocolate is rich in polyphenols, naturally occurring compounds also found in fruits and vegetables, such as grapes, apples and berries. Research has shown that consuming plant polyphenols can be protective against cancer, diabetes and osteoporosis as well as cardiovascular and neurodegenerative diseases.
“So keeping the healthy part of it and reducing the oily part of it, which is not healthy, but is giving you that indulgence of it … that was the final aim,” Soltanahmadi says. He adds that the team has been approached by individuals in the chocolate industry about their research. “Everyone wants to have a healthy chocolate, which at the same time tastes brilliant and gives you that self-indulging experience.”
Probiotic bacteria can be engineered to fight antibiotic-resistant superbugs by releasing chemicals that kill them.
In 1945, almost two decades after Alexander Fleming discovered penicillin, he warned that as antibiotics use grows, they may lose their efficiency. He was prescient—the first case of penicillin resistance was reported two years later. Back then, not many people paid attention to Fleming’s warning. After all, the “golden era” of the antibiotics age had just began. By the 1950s, three new antibiotics derived from soil bacteria — streptomycin, chloramphenicol, and tetracycline — could cure infectious diseases like tuberculosis, cholera, meningitis and typhoid fever, among others.
Today, these antibiotics and many of their successors developed through the 1980s are gradually losing their effectiveness. The extensive overuse and misuse of antibiotics led to the rise of drug resistance. The livestock sector buys around 80 percent of all antibiotics sold in the U.S. every year. Farmers feed cows and chickens low doses of antibiotics to prevent infections and fatten up the animals, which eventually causes resistant bacterial strains to evolve. If manure from cattle is used on fields, the soil and vegetables can get contaminated with antibiotic-resistant bacteria. Another major factor is doctors overprescribing antibiotics to humans, particularly in low-income countries. Between 2000 to 2018, the global rates of human antibiotic consumption shot up by 46 percent.
In recent years, researchers have been exploring a promising avenue: the use of synthetic biology to engineer new bacteria that may work better than antibiotics. The need continues to grow, as a Lancet study linked antibiotic resistance to over 1.27 million deaths worldwide in 2019, surpassing HIV/AIDS and malaria. The western sub-Saharan Africa region had the highest death rate (27.3 people per 100,000).
Researchers warn that if nothing changes, by 2050, antibiotic resistance could kill 10 million people annually.
To make it worse, our remedy pipelines are drying up. Out of the 18 biggest pharmaceutical companies, 15 abandoned antibiotic development by 2013. According to the AMR Action Fund, venture capital has remained indifferent towards biotech start-ups developing new antibiotics. In 2019, at least two antibiotic start-ups filed for bankruptcy. As of December 2020, there were 43 new antibiotics in clinical development. But because they are based on previously known molecules, scientists say they are inadequate for treating multidrug-resistant bacteria. Researchers warn that if nothing changes, by 2050, antibiotic resistance could kill 10 million people annually.
The rise of synthetic biology
To circumvent this dire future, scientists have been working on alternative solutions using synthetic biology tools, meaning genetically modifying good bacteria to fight the bad ones.
From the time life evolved on earth around 3.8 billion years ago, bacteria have engaged in biological warfare. They constantly strategize new methods to combat each other by synthesizing toxic proteins that kill competition.
For example, Escherichia coli produces bacteriocins or toxins to kill other strains of E.coli that attempt to colonize the same habitat. Microbes like E.coli (which are not all pathogenic) are also naturally present in the human microbiome. The human microbiome harbors up to 100 trillion symbiotic microbial cells. The majority of them are beneficial organisms residing in the gut at different compositions.
The chemicals that these “good bacteria” produce do not pose any health risks to us, but can be toxic to other bacteria, particularly to human pathogens. For the last three decades, scientists have been manipulating bacteria’s biological warfare tactics to our collective advantage.
In the late 1990s, researchers drew inspiration from electrical and computing engineering principles that involve constructing digital circuits to control devices. In certain ways, every cell in living organisms works like a tiny computer. The cell receives messages in the form of biochemical molecules that cling on to its surface. Those messages get processed within the cells through a series of complex molecular interactions.
Synthetic biologists can harness these living cells’ information processing skills and use them to construct genetic circuits that perform specific instructions—for example, secrete a toxin that kills pathogenic bacteria. “Any synthetic genetic circuit is merely a piece of information that hangs around in the bacteria’s cytoplasm,” explains José Rubén Morones-Ramírez, a professor at the Autonomous University of Nuevo León, Mexico. Then the ribosome, which synthesizes proteins in the cell, processes that new information, making the compounds scientists want bacteria to make. “The genetic circuit remains separated from the living cell’s DNA,” Morones-Ramírez explains. When the engineered bacteria replicates, the genetic circuit doesn’t become part of its genome.
Highly intelligent by bacterial standards, some multidrug resistant V. cholerae strains can also “collaborate” with other intestinal bacterial species to gain advantage and take hold of the gut.
In 2000, Boston-based researchers constructed an E.coli with a genetic switch that toggled between turning genes on and off two. Later, they built some safety checks into their bacteria. “To prevent unintentional or deleterious consequences, in 2009, we built a safety switch in the engineered bacteria’s genetic circuit that gets triggered after it gets exposed to a pathogen," says James Collins, a professor of biological engineering at MIT and faculty member at Harvard University’s Wyss Institute. “After getting rid of the pathogen, the engineered bacteria is designed to switch off and leave the patient's body.”
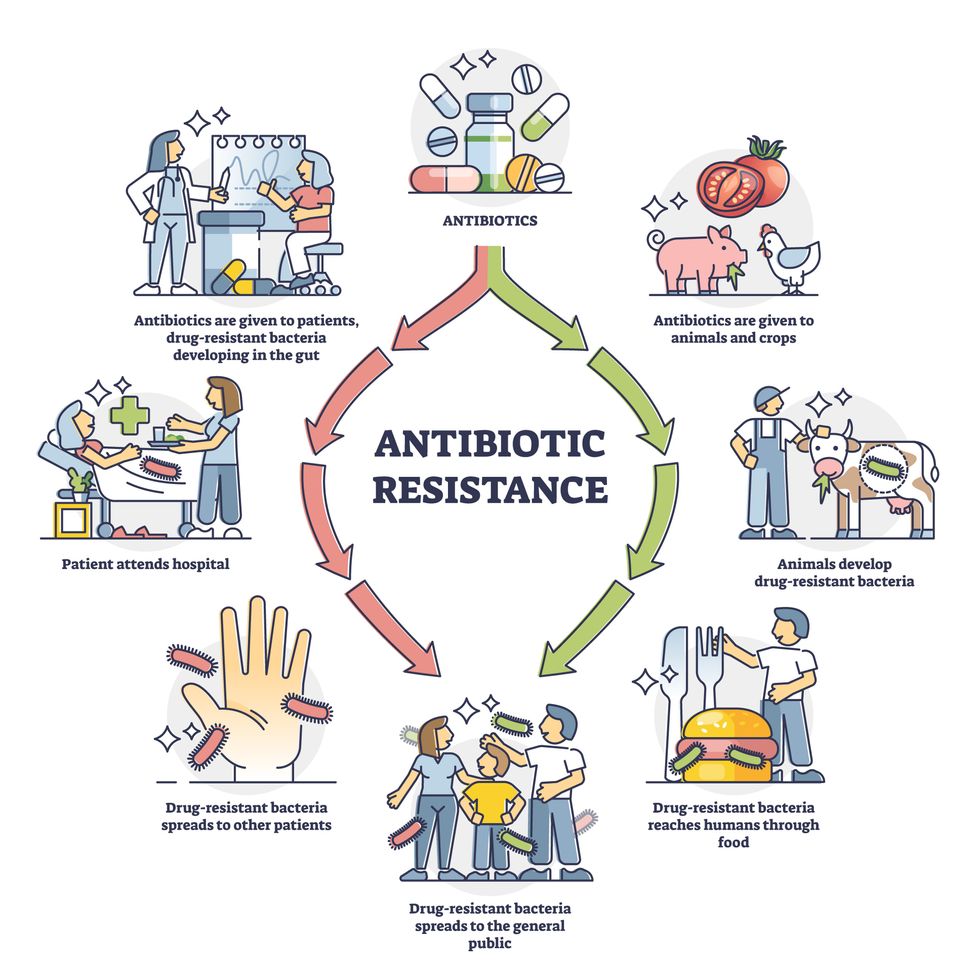
Overuse and misuse of antibiotics causes resistant strains to evolve
Adobe Stock
Seek and destroy
As the field of synthetic biology developed, scientists began using engineered bacteria to tackle superbugs. They first focused on Vibrio cholerae, which in the 19th and 20th century caused cholera pandemics in India, China, the Middle East, Europe, and Americas. Like many other bacteria, V. cholerae communicate with each other via quorum sensing, a process in which the microorganisms release different signaling molecules, to convey messages to its brethren. Highly intelligent by bacterial standards, some multidrug resistant V. cholerae strains can also “collaborate” with other intestinal bacterial species to gain advantage and take hold of the gut. When untreated, cholera has a mortality rate of 25 to 50 percent and outbreaks frequently occur in developing countries, especially during floods and droughts.
Sometimes, however, V. cholerae makes mistakes. In 2008, researchers at Cornell University observed that when quorum sensing V. cholerae accidentally released high concentrations of a signaling molecule called CAI-1, it had a counterproductive effect—the pathogen couldn’t colonize the gut.
So the group, led by John March, professor of biological and environmental engineering, developed a novel strategy to combat V. cholerae. They genetically engineered E.coli to eavesdrop on V. cholerae communication networks and equipped it with the ability to release the CAI-1 molecules. That interfered with V. cholerae progress. Two years later, the Cornell team showed that V. cholerae-infected mice treated with engineered E.coli had a 92 percent survival rate.
These findings inspired researchers to sic the good bacteria present in foods like yogurt and kimchi onto the drug-resistant ones.
Three years later in 2011, Singapore-based scientists engineered E.coli to detect and destroy Pseudomonas aeruginosa, an often drug-resistant pathogen that causes pneumonia, urinary tract infections, and sepsis. Once the genetically engineered E.coli found its target through its quorum sensing molecules, it then released a peptide, that could eradicate 99 percent of P. aeruginosa cells in a test-tube experiment. The team outlined their work in a Molecular Systems Biology study.
“At the time, we knew that we were entering new, uncharted territory,” says lead author Matthew Chang, an associate professor and synthetic biologist at the National University of Singapore and lead author of the study. “To date, we are still in the process of trying to understand how long these microbes stay in our bodies and how they might continue to evolve.”
More teams followed the same path. In a 2013 study, MIT researchers also genetically engineered E.coli to detect P. aeruginosa via the pathogen’s quorum-sensing molecules. It then destroyed the pathogen by secreting a lab-made toxin.
Probiotics that fight
A year later in 2014, a Nature study found that the abundance of Ruminococcus obeum, a probiotic bacteria naturally occurring in the human microbiome, interrupts and reduces V.cholerae’s colonization— by detecting the pathogen’s quorum sensing molecules. The natural accumulation of R. obeum in Bangladeshi adults helped them recover from cholera despite living in an area with frequent outbreaks.
The findings from 2008 to 2014 inspired Collins and his team to delve into how good bacteria present in foods like yogurt and kimchi can attack drug-resistant bacteria. In 2018, Collins and his team developed the engineered probiotic strategy. They tweaked a bacteria commonly found in yogurt called Lactococcus lactis to treat cholera.
Engineered bacteria can be trained to target pathogens when they are at their most vulnerable metabolic stage in the human gut. --José Rubén Morones-Ramírez.
More scientists followed with more experiments. So far, researchers have engineered various probiotic organisms to fight pathogenic bacteria like Staphylococcus aureus (leading cause of skin, tissue, bone, joint and blood infections) and Clostridium perfringens (which causes watery diarrhea) in test-tube and animal experiments. In 2020, Russian scientists engineered a probiotic called Pichia pastoris to produce an enzyme called lysostaphin that eradicated S. aureus in vitro. Another 2020 study from China used an engineered probiotic bacteria Lactobacilli casei as a vaccine to prevent C. perfringens infection in rabbits.
In a study last year, Ramírez’s group at the Autonomous University of Nuevo León, engineered E. coli to detect quorum-sensing molecules from Methicillin-resistant Staphylococcus aureus or MRSA, a notorious superbug. The E. coli then releases a bacteriocin that kills MRSA. “An antibiotic is just a molecule that is not intelligent,” says Ramírez. “On the other hand, engineered bacteria can be trained to target pathogens when they are at their most vulnerable metabolic stage in the human gut.”
Collins and Timothy Lu, an associate professor of biological engineering at MIT, found that engineered E. coli can help treat other conditions—such as phenylketonuria, a rare metabolic disorder, that causes the build-up of an amino acid phenylalanine. Their start-up Synlogic aims to commercialize the technology, and has completed a phase 2 clinical trial.
Circumventing the challenges
The bacteria-engineering technique is not without pitfalls. One major challenge is that beneficial gut bacteria produce their own quorum-sensing molecules that can be similar to those that pathogens secrete. If an engineered bacteria’s biosensor is not specific enough, it will be ineffective.
Another concern is whether engineered bacteria might mutate after entering the gut. “As with any technology, there are risks where bad actors could have the capability to engineer a microbe to act quite nastily,” says Collins of MIT. But Collins and Ramírez both insist that the chances of the engineered bacteria mutating on its own are virtually non-existent. “It is extremely unlikely for the engineered bacteria to mutate,” Ramírez says. “Coaxing a living cell to do anything on command is immensely challenging. Usually, the greater risk is that the engineered bacteria entirely lose its functionality.”
However, the biggest challenge is bringing the curative bacteria to consumers. Pharmaceutical companies aren’t interested in antibiotics or their alternatives because it’s less profitable than developing new medicines for non-infectious diseases. Unlike the more chronic conditions like diabetes or cancer that require long-term medications, infectious diseases are usually treated much quicker. Running clinical trials are expensive and antibiotic-alternatives aren’t lucrative enough.
“Unfortunately, new medications for antibiotic resistant infections have been pushed to the bottom of the field,” says Lu of MIT. “It's not because the technology does not work. This is more of a market issue. Because clinical trials cost hundreds of millions of dollars, the only solution is that governments will need to fund them.” Lu stresses that societies must lobby to change how the modern healthcare industry works. “The whole world needs better treatments for antibiotic resistance.”