An Astounding Treatment at an Astounding Price: Who Gets to Benefit?

Tony and Kelly Mantoan, with their boys Teddy and Fulton, who both suffer from SMA, a genetic disorder that makes walking, swallowing, and breathing progressively difficult.
Kelly Mantoan was nursing her newborn son, Teddy, in the NICU in a Philadelphia hospital when her doctor came in and silently laid a hand on her shoulder. Immediately, Kelly knew what the gesture meant and started to sob: Teddy, like his one-year-old brother, Fulton, had just tested positive for a neuromuscular condition called spinal muscular atrophy (SMA).
The boys were 8 and 10 when Kelly heard about an experimental new treatment, still being tested in clinical trials, called Spinraza.
"We knew that [SMA] was a genetic disorder, and we knew that we had a 1 in 4 chance of Teddy having SMA," Mantoan recalls. But the idea of having two children with the same severe disability seemed too unfair for Kelly and her husband, Tony, to imagine. "We had lots of well-meaning friends tell us, well, God won't do this to you twice," she says. Except that He, or a cruel trick of nature, had.
In part, the boys' diagnoses were so devastating because there was little that could be done at the time, back in 2009 and 2010, when the boys were diagnosed. Affecting an estimated 1 in 11,000 babies, SMA is a degenerative disease in which the body is deficient in survival motor neuron (SMN) protein, thanks to a genetic mutation or absence of the body's SNM1 gene. So muscles that control voluntary movement – such as walking, breathing, and swallowing – weaken and eventually cease to function altogether.
Babies diagnosed with SMA Type 1 rarely live past toddlerhood, while people diagnosed with SMA Types 2, 3, and 4 can live into adulthood, usually with assistance like ventilators and feeding tubes. Shortly after birth, both Teddy Mantoan and his brother, Fulton, were diagnosed with SMA Type 2.
The boys were 8 and 10 when Kelly heard about an experimental new treatment, still being tested in clinical trials, called Spinraza. Up until then, physical therapy was the only sanctioned treatment for SMA, and Kelly enrolled both her boys in weekly sessions to preserve some of their muscle strength as the disease marched forward. But Spinraza – a grueling regimen of lumbar punctures and injections designed to stimulate a backup survival motor neuron gene to produce more SMN protein – offered new hope.
In clinical trials, after just a few doses of Spinraza, babies with SMA Type 1 began meeting normal developmental milestones – holding up their heads, rolling over, and sitting up. In other trials, Spinraza treatment delayed the need for permanent ventilation, while patients on the placebo arm continued to lose function, and several died. Spinraza was such a success, and so well tolerated among patients, that clinical trials ended early and the drug was fast-tracked for FDA approval in 2016. In January 2017, when Kelly got the call that Fulton and Teddy had been approved by the hospital to start Spinraza infusions, Kelly dropped to her knees in the middle of the kitchen and screamed.
Spinraza, manufactured by Biogen, has been hailed as revolutionary, but it's also not without drawbacks: Priced per injection, just one dose of Spinraza costs $125,000, making it one of the most expensive drugs on the global market. What's worse, treatment requires a "loading dose" of four injections over a four-week period, and then periodic injections every four months, indefinitely. For the first year of treatment, Spinraza treatment costs $750,000 – and then $375,000 for every year thereafter.
Last week, a competitive treatment for SMA Type 1 manufactured by Novartis burst onto the market. The new treatment, called Zolgensma, is a one-time gene therapy intended to be given to infants and is currently priced at $2.125 million, or $425,000 annually for five years, making it the most expensive drug in the world. Like Spinraza, Zolgensma is currently raising challenging questions about how insurers and government payers like Medicaid will be able to afford these treatments without bankrupting an already-strained health care system.
To Biogen's credit, the company provides financial aid for Spinraza patients with private insurance who pay co-pays for treatment, as well as for those who have been denied by Medicaid and Medicare. But getting insurance companies to agree to pay for Spinraza can often be an ordeal in itself. Although Fulton and Teddy Mantoan were approved for treatment over two years ago, a lengthy insurance battle delayed treatment for another eight months – time that, for some SMA patients, can mean a significant loss of muscular function.
Kelly didn't notice anything in either boy – positive or negative – for the first few months of Spinraza injections. But one day in November 2017, as Teddy was lowered off his school bus in his wheelchair, he turned to say goodbye to his friends and "dab," – a dance move where one's arms are extended briefly across the chest and in the air. Normally, Teddy would dab by throwing his arms up in the air with momentum, striking a pose quickly before they fell down limp at his sides. But that day, Teddy held his arms rigid in the air. His classmates, along with Kelly, were stunned. "Teddy, look at your arms!" Kelly remembers shrieking. "You're holding them up – you're dabbing!"

Teddy and Fulton Mantoan, who both suffer from spinal muscular atrophy, have seen life-changing results from Spinraza.
(Courtesy of Kelly Mantoan)
Not long after Teddy's dab, the Mantoans started seeing changes in Fulton as well. "With Fulton, we realized suddenly that he was no longer choking on his food during meals," Kelly said. "Almost every meal we'd have to stop and have him take a sip of water and make him slow down and take small bites so he wouldn't choke. But then we realized we hadn't had to do that in a long time. The nurses at school were like, 'it's not an issue anymore.'"
For the Mantoans, this was an enormous relief: Less choking meant less chance of aspiration pneumonia, a leading cause of death for people with SMA Types 1 and 2.
While Spinraza has been life-changing for the Mantoans, it remains painfully out of reach for many others. Thanks to Spinraza's enormous price tag, the threshold for who gets to use it is incredibly high: Adult and pediatric patients, particularly those with state-sponsored insurance, have reported multiple insurance denials, lengthy appeals processes, and endless bureaucracy from insurance and hospitals alike that stand in the way of treatment.
Kate Saldana, a 21-year-old woman with Type 2 SMA, is one of the many adult patients who have been lobbying for the drug. Saldana, who uses a ventilator 20 hours each day, says that Medicaid denied her Spinraza treatments because they mistakenly believed that she used a ventilator full-time. Saldana is currently in the process of appealing their decision, but knows she is fighting an uphill battle.

Kate Saldana, who suffers from Type 2 SMA, has been fighting unsuccessfully for Medicaid to cover Spinraza.
(Courtesy of Saldana)
"Originally, the treatments were studied and created for infants and children," Saldana said in an e-mail. "There is a plethora of data to support the effectiveness of Spinraza in those groups, but in adults it has not been studied as much. That makes it more difficult for insurance to approve it, because they are not sure if it will be as beneficial."
Saldana has been pursuing treatment unsuccessfully since last August – but others, like Kimberly Hill, a 32-year-old with SMA Type 2, have been waiting even longer. Hill, who lives in Oklahoma, has been fighting for treatment since Spinraza went on the U.S. market in December 2016. Because her mobility is limited to the use of her left thumb, Hill is eager to try anything that will enable her to keep working and finish a Master's degree in Fire and Emergency Management.
"Obviously, my family and I were elated with the approval of Spinraza," Hill said in an e-mail. "We thought I would finally have the chance to get a little stronger and healthier." But with Medicare and Medicaid, coverage and eligibility varies wildly by state. Earlier this year, Medicaid approved Spinraza for adult patients only if a clawback clause was attached to the approval, meaning that under certain conditions the Medicaid funds would need to be paid back. Because of the clawback clause, hospitals have been reluctant to take on Spinraza treatments, effectively barring adult Medicaid patients from accessing the drug altogether.
Hill's hospital is currently in negotiations with Medicaid to move forward with Spinraza treatment, but in the meantime, Hill is in limbo. "We keep being told there is nothing we can do, and we are devastated," Hill said.
"I felt extremely sad and honestly a bit forgotten, like adults [with SMA] don't matter."
Between Spinraza and its new competitor, Zolgensma, some are speculating that insurers will start to favor Zolgensma coverage instead, since the treatment is shorter and ultimately cheaper than Spinraza in the long term. But for some adults with SMA who can't access Spinraza and who don't qualify for Zolgensma treatment, the issue of what insurers will cover is moot.
"I was so excited when I heard that Zolgensma was approved by the FDA," said Annie Wilson, an adult SMA patient from Alameda, Calif. who has been fighting for Spinraza since 2017. "When I became aware that it was only being offered to children, I felt extremely sad and honestly a bit forgotten, like adults [with SMA] don't matter."
According to information from a Biogen representative, more than 7500 people worldwide have been treated with Spinraza to date, one third of whom are adults.
While Spinraza has been revolutionary for thousands of patients, it's unclear how many more lives state agencies and insurance companies will allow it to save.
Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
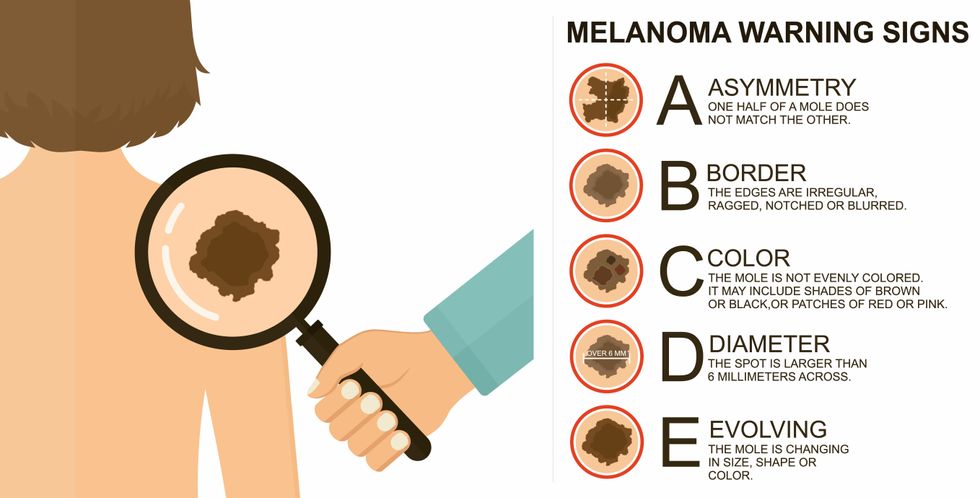
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.

