Anti-Aging Pioneer Aubrey de Grey: “People in Middle Age Now Have a Fair Chance”
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

Aubrey de Grey at the World Stem Cell Summit in Miami on January 25, 2018.
Aging is not a mystery, says famed researcher Dr. Aubrey de Grey, perhaps the world's foremost advocate of the provocative view that medical technology will one day allow humans to control the aging process and live healthily into our hundreds—or even thousands.
"The cultural attitudes toward all of this are going to be completely turned upside down by sufficiently promising results in the lab, in mice."
He likens aging to a car wearing down over time; as the body operates normally, it accumulates damage which can be tolerated for a while, but eventually sends us into steep decline. The most promising way to escape this biological reality, he says, is to repair the damage as needed with precise scientific tools.
The bad news is that doing this groundbreaking research takes a long time and a lot of money, which has not always been readily available, in part due to a cultural phenomenon he terms "the pro-aging trance." Cultural attitudes have long been fatalistic about the inevitability of aging; many people balk at the seemingly implausible prospect of indefinite longevity.
But the good news for de Grey—and those who are cheering him on—is that his view is becoming less radical these days. Both the academic and private sectors are racing to tackle aging; his own SENS Research Foundation, for one, has spun out into five different companies. Defeating aging, he says, "is not just a future industry; it's an industry now that will be both profitable and extremely good for your health."
De Grey sat down with Editor-in-Chief Kira Peikoff at the World Stem Cell Summit in Miami to give LeapsMag the latest scoop on his work. Here is an edited and condensed version of our conversation.
Since your book Ending Aging was published a decade ago, scientific breakthroughs in stem cell research, genome editing, and other fields have taken the world by storm. Which of these have most affected your research?
They have all affected it a lot in one way, and hardly at all in another way. They have speeded it up--facilitated short cuts, ways to get where we're already trying to go. What they have not done is identified any fundamental changes to the overall strategy. In the book, we described the seven major types of damage, and particular ways of going about fixing each of them, and that hasn't changed.
"Repair at the microscopic level, one would be able to expect to do without surgery, just by injecting the right kind of stem cells."
Has any breakthrough specifically made the biggest impact?
It's not just the obvious things, like iPS (induced pluripotent stem cells) and CRISPR (a precise tool for editing genes). It's also the more esoteric things that applied specifically to certain of our areas, but most people don't really know about them. For example, the identification of how to control something called co-translational mitochondrial protein import.
How much of the future of anti-aging treatments will involve regeneration of old tissue, or wholesale growth of new organs?
The more large-scale ones, regenerating whole new organs, are probably only going to play a role in the short-term and will be phased out relatively rapidly, simply because, in order to be useful, one has to employ surgery, which is really invasive. We'll want to try to get around that, but it seems quite likely that in the very early stages, the techniques we have for repairing things at the molecular and cellular level in situ will be insufficiently comprehensive, and so we will need to do the more sledgehammer approach of building a whole new organ and sticking it in.
Every time you are in a position where you're replacing an organ, you have the option, in principle, of repairing the organ, without replacing it. And repair at the microscopic level, one would be able to expect to do without surgery, just by injecting the right kind of stem cells or whatever. That would be something one would expect to be able to apply to someone much closer to death's door and much more safely in general, and probably much more cheaply. One would expect that subsequent generations of these therapies would move in that direction.
Your foundation is working on an initiative requiring $50 million in funding—
Well, if we had $50 million per year in funding, we could go about three times faster than we are on $5 million per year.
And you're looking at a 2021 timeframe to start human trials?
That's approximate. Remember, because we accumulate in the body so many different types of damage, that means we have many different types of therapy to repair that damage. And of course, each of those types has to be developed independently. It's very much a divide and conquer therapy. The therapies interact with each other to some extent; the repair of one type of damage may slow down the creation of another type of damage, but still that's how it's going to be.
And some of these therapies are much easier to implement than others. The easier components of what we need to do are already in clinical trials—stem cell therapies especially, and immunotherapy against amyloid in the brain, for example. Even in phase III clinical trials in some cases. So when I talk about a timeframe like 2021, or early 20s shall we say, I'm really talking about the most difficult components.
What recent strides are you most excited about?
Looking back over the past couple of years, I'm particularly proud of the successes we've had in the very most difficult areas. If you go through the 7 components of SENS, there are two that have absolutely been stuck in a rut and have gotten nowhere for 15 to 20 years, and we basically fixed that in both cases. We published two years ago in Science magazine that essentially showed a way forward against the stiffening of the extracellular matrix, which is responsible for things like wrinkles and hypertension. And then a year ago, we published a real breakthrough paper with regard to placing copies of the mitochondria DNA in the nuclear DNA modified in such a way that they still work, which is an idea that had been around for 30 years; everyone had given up on it, some a long time ago, and we basically revived it.
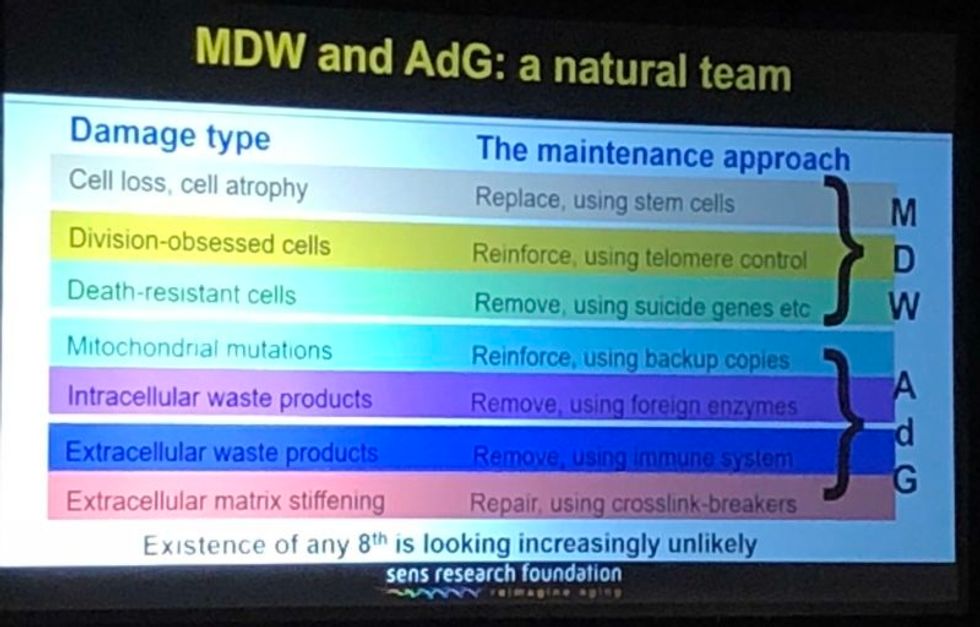
A slide presented by Aubrey de Grey, referencing his collaboration with Mike West at AgeX, showing the 7 types of damage that he believes must be repaired to end aging.
(Courtesy Kira Peikoff)
That's exciting. What do you think are the biggest barriers to defeating aging today: the technological challenges, the regulatory framework, the cost, or the cultural attitude of the "pro-aging" trance?
One can't really address those independently of each other. The technological side is one thing; it's hard, but we know where we're going, we've got a plan. The other ones are very intertwined with each other. A lot of people are inclined to say, the regulatory hurdle will be completely insurmountable, plus people don't recognize aging as a disease, so it's going to be a complete nonstarter. I think that's nonsense. And the reason is because the cultural attitudes toward all of this are going to be completely turned upside down before we have to worry about the regulatory hurdles. In other words, they're going to be turned upside down by sufficiently promising results in the lab, in mice. Once we get to be able to rejuvenate actually old mice really well so they live substantially longer than they otherwise would have done, in a healthy state, everyone's going to know about it and everyone's going to demand – it's not going to be possible to get re-elected unless you have a manifesto commitment to turn the FDA completely upside down and make sure this happens without any kind of regulatory obstacle.
I've been struggling away all these years trying to bring little bits of money in the door, and the reason I have is because of the skepticism as to regards whether this could actually work, combined with the pro-aging trance, which is a product of the skepticism – people not wanting to get their hopes up, so finding excuses about aging being a blessing in disguise, so they don't have to think about it. All of that will literally disintegrate pretty much overnight when we have the right kind of sufficiently impressive progress in the lab. Therefore, the availability of money will also [open up]. It's already cracking: we're already seeing the beginnings of the actual rejuvenation biotechnology industry that I've been talking about with a twinkle in my eye for some years.
"For humans, a 50-50 chance would be twenty years at this point, and there's a 10 percent chance that we won't get there for a hundred years."
Why do you think the culture is starting to shift?
There's no one thing yet. There will be that tipping point I mentioned, perhaps five years from now when we get a real breakthrough, decisive results in mice that make it simply impossible to carry on being fatalistic about all this. Prior to that, what we're already seeing is the impact of sheer old-school repeat advertising—me going out there, banging away and saying the same fucking thing again and again, and nobody saying anything that persuasively knocks me down. … And it's also the fact that we are making incremental amounts of progress, not just ourselves, but the scientific community generally. It has become incrementally more plausible that what I say might be true.
I'm sure you hate getting the timeline question, but if we're five years away from this breakthrough in mice, it's hard to resist asking—how far is that in terms of a human cure?
When I give any kind of timeframes, the only real care I have to take is to emphasize the variance. In this case I think we have got a 50-50 chance of getting to that tipping point in mice within five years from now, certainly it could be 10 or 15 years if we get unlucky. Similarly, for humans, a 50-50 chance would be twenty years at this point, and there's a 10 percent chance that we won't get there for a hundred years.
"I don't get people coming to me saying, well I don't think medicine for the elderly should be done because if it worked it would be a bad thing. People like to ignore this contradiction."
What would you tell skeptical people are the biggest benefits of a very long-lived population?
Any question about the longevity of people is the wrong question. Because the longevity that people fixate about so much will only ever occur as a side effect of health. However long ago you were born or however recently, if you're sick, you're likely to die fairly soon unless we can stop you being sick. Whereas if you're healthy, you're not. So if we do as well as we think we can do in terms of keeping people healthy and youthful however long ago they were born, then the side effect in terms of longevity and life expectancy is likely to be very large. But it's still a side effect, so the way that people actually ought to be—in fact have a requirement to be—thinking, is about whether they want people to be healthy.
Now I don't get people coming to me saying, well I don't think medicine for the elderly should be done because if it worked it would be a bad thing. People like to ignore this contradiction, they like to sweep it under the carpet and say, oh yeah, aging is totally a good thing.
People will never actually admit to the fact that what they are fundamentally saying is medicine for the elderly, if it actually works, would be bad, but still that is what they are saying.
Shifting gears a bit, I'm curious to find out which other radical visionaries in science and tech today you most admire?
Fair question. One is Mike West. I have the great privilege that I now work for him part-time with Age X. I have looked up to him very much for the past ten years, because what he did over the past 20 years starting with Geron is unimaginable today. He was working in an environment where I would not have dreamt of the possibility of getting any private money, any actual investment, in something that far out, that far ahead of its time, and he did it, again and again. It's insane what he managed to do.
What about someone like Elon Musk?
Sure, he's another one. He is totally impervious to the caution and criticism and conservatism that pervades humanity, and he's getting on making these bloody self-driving cars, space tourism, and so on, making them happen. He's thinking just the way I'm thinking really.
"You can just choose how frequently and how thoroughly you repair the damage. And you can make a different choice next time."
You famously said ten years ago that you think the first person to live to 1000 is already alive. Do you think that's still the case?
Definitely, yeah. I can't see how it could not be. Again, it's a probabilistic thing. I said there's at least a 10 percent chance that we won't get to what I call Longevity Escape Velocity for 100 years and if that's true, then the statement about 1000 years being alive already is not going to be the case. But for sure, I believe that the beneficiaries of what we may as well call SENS 1.0, the point where we get to LEV, those people are exceptionally unlikely ever to suffer from any kind of ill health correlated with their age. Because we will never fall below Longevity Escape Velocity once we attain it.
Could someone who was just born today expect—
I would say people in middle age now have a fair chance. Remember – a 50/50 chance of getting to LEV within 20 years, and when you get there, you don't just stay at biologically 70 or 80, you are rejuvenated back to biologically 30 or 40 and you stay there, so your risk of death each year is not related to how long ago you were born, it's the same as a young adult. Today, that's less than 1 in 1000 per year, and that number is going to go down as we get self-driving cars and all that, so actually 1000 is a very conservative number.
So you would be able to choose what age you wanted to go back to?
Oh sure, of course, it's just like a car. What you're doing is you're repairing damage, and the damage is still being created by the body's metabolism, so you can just choose how frequently and how thoroughly you repair the damage. And you can make a different choice next time.
What would be your perfect age?
I have no idea. That's something I don't have an opinion about, because I could change it whenever I like.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
The future of non-hormonal birth control: Antibodies can stop sperm in their tracks
Many women want non-hormonal birth control. A 22-year-old's findings were used to launch a company that could, within the decade, bring a new kind of contraceptive to the marketplace.
Unwanted pregnancy can now be added to the list of preventions that antibodies may be fighting in the near future. For decades, really since the 1980s, engineered monoclonal antibodies have been knocking out invading germs — preventing everything from cancer to COVID. Sperm, which have some of the same properties as germs, may be next.
Not only is there an unmet need on the market for alternatives to hormonal contraceptives, the genesis for the original research was personal for the then 22-year-old scientist who led it. Her findings were used to launch a company that could, within the decade, bring a new kind of contraceptive to the marketplace.
The genesis
It’s Suruchi Shrestha’s research — published in Science Translational Medicine in August 2021 and conducted as part of her dissertation while she was a graduate student at the University of North Carolina at Chapel Hill — that could change the future of contraception for many women worldwide. According to a Guttmacher Institute report, in the U.S. alone, there were 46 million sexually active women of reproductive age (15–49) who did not want to get pregnant in 2018. With the overturning of Roe v. Wade last year, Shrestha’s research could, indeed, be life changing for millions of American women and their families.
Now a scientist with NextVivo, Shrestha is not directly involved in the development of the contraceptive that is based on her research. But, back in 2016 when she was going through her own problems with hormonal contraceptives, she “was very personally invested” in her research project, Shrestha says. She was coping with a long list of negative effects from an implanted hormonal IUD. According to the Mayo Clinic, those can include severe pelvic pain, headaches, acute acne, breast tenderness, irregular bleeding and mood swings. After a year, she had the IUD removed, but it took another full year before all the side effects finally subsided; she also watched her sister suffer the “same tribulations” after trying a hormonal IUD, she says.
For contraceptive use either daily or monthly, Shrestha says, “You want the antibody to be very potent and also cheap.” That was her goal when she launched her study.
Shrestha unshelved antibody research that had been sitting idle for decades. It was in the late 80s that scientists in Japan first tried to develop anti-sperm antibodies for contraceptive use. But, 35 years ago, “Antibody production had not been streamlined as it is now, so antibodies were very expensive,” Shrestha explains. So, they shifted away from birth control, opting to focus on developing antibodies for vaccines.
Over the course of the last three decades, different teams of researchers have been working to make the antibody more effective, bringing the cost down, though it’s still expensive, according to Shrestha. For contraceptive use either daily or monthly, she says, “You want the antibody to be very potent and also cheap.” That was her goal when she launched her study.
The problem
The problem with contraceptives for women, Shrestha says, is that all but a few of them are hormone-based or have other negative side effects. In fact, some studies and reports show that millions of women risk unintended pregnancy because of medical contraindications with hormone-based contraceptives or to avoid the risks and side effects. While there are about a dozen contraceptive choices for women, there are two for men: the condom, considered 98% effective if used correctly, and vasectomy, 99% effective. Neither of these choices are hormone-based.
On the non-hormonal side for women, there is the diaphragm which is considered only 87 percent effective. It works better with the addition of spermicides — Nonoxynol-9, or N-9 — however, they are detergents; they not only kill the sperm, they also erode the vaginal epithelium. And, there’s the non-hormonal IUD which is 99% effective. However, the IUD needs to be inserted by a medical professional, and it has a number of negative side effects, including painful cramping at a higher frequency and extremely heavy or “abnormal” and unpredictable menstrual flows.
The hormonal version of the IUD, also considered 99% effective, is the one Shrestha used which caused her two years of pain. Of course, there’s the pill, which needs to be taken daily, and the birth control ring which is worn 24/7. Both cause side effects similar to the other hormonal contraceptives on the market. The ring is considered 93% effective mostly because of user error; the pill is considered 99% effective if taken correctly.
“That’s where we saw this opening or gap for women. We want a safe, non-hormonal contraceptive,” Shrestha says. Compounding the lack of good choices, is poor access to quality sex education and family planning information, according to the non-profit Urban Institute. A focus group survey suggested that the sex education women received “often lacked substance, leaving them feeling unprepared to make smart decisions about their sexual health and safety,” wrote the authors of the Urban Institute report. In fact, nearly half (45%, or 2.8 million) of the pregnancies that occur each year in the US are unintended, reports the Guttmacher Institute. Globally the numbers are similar. According to a new report by the United Nations, each year there are 121 million unintended pregnancies, worldwide.
The science
The early work on antibodies as a contraceptive had been inspired by women with infertility. It turns out that 9 to 12 percent of women who are treated for infertility have antibodies that develop naturally and work against sperm. Shrestha was encouraged that the antibodies were specific to the target — sperm — and therefore “very safe to use in women.” She aimed to make the antibodies more stable, more effective and less expensive so they could be more easily manufactured.
Since antibodies tend to stick to things that you tell them to stick to, the idea was, basically, to engineer antibodies to stick to sperm so they would stop swimming. Shrestha and her colleagues took the binding arm of an antibody that they’d isolated from an infertile woman. Then, targeting a unique surface antigen present on human sperm, they engineered a panel of antibodies with as many as six to 10 binding arms — “almost like tongs with prongs on the tongs, that bind the sperm,” explains Shrestha. “We decided to add those grabbers on top of it, behind it. So it went from having two prongs to almost 10. And the whole goal was to have so many arms binding the sperm that it clumps it” into a “dollop,” explains Shrestha, who earned a patent on her research.

Suruchi Shrestha works in the lab with a colleague. In 2016, her research on antibodies for birth control was inspired by her own experience with side effects from an implanted hormonal IUD.
UNC - Chapel Hill
The sperm stays right where it met the antibody, never reaching the egg for fertilization. Eventually, and naturally, “Our vaginal system will just flush it out,” Shrestha explains.
“She showed in her early studies that [she] definitely got the sperm immotile, so they didn't move. And that was a really promising start,” says Jasmine Edelstein, a scientist with an expertise in antibody engineering who was not involved in this research. Shrestha’s team at UNC reproduced the effect in the sheep, notes Edelstein, who works at the startup Be Biopharma. In fact, Shrestha’s anti-sperm antibodies that caused the sperm to agglutinate, or clump together, were 99.9% effective when delivered topically to the sheep’s reproductive tracts.
The future
Going forward, Shrestha thinks the ideal approach would be delivering the antibodies through a vaginal ring. “We want to use it at the source of the spark,” Shrestha says, as opposed to less direct methods, such as taking a pill. The ring would dissolve after one month, she explains, “and then you get another one.”
Engineered to have a long shelf life, the anti-sperm antibody ring could be purchased without a prescription, and women could insert it themselves, without a doctor. “That's our hope, so that it is accessible,” Shrestha says. “Anybody can just go and grab it and not worry about pregnancy or unintended pregnancy.”
Her patented research has been licensed by several biotech companies for clinical trials. A number of Shrestha’s co-authors, including her lab advisor, Sam Lai, have launched a company, Mucommune, to continue developing the contraceptives based on these antibodies.
And, results from a small clinical trial run by researchers at Boston University Chobanian & Avedisian School of Medicine show that a dissolvable vaginal film with antibodies was safe when tested on healthy women of reproductive age. That same group of researchers last year received a $7.2 million grant from the National Institute of Health for further research on monoclonal antibody-based contraceptives, which have also been shown to block transmission of viruses, like HIV.
“As the costs come down, this becomes a more realistic option potentially for women,” says Edelstein. “The impact could be tremendous.”
This article was first published by Leaps.org in December, 2022. It has been lightly edited with updates for timeliness.
Researchers probe extreme gene therapy for severe alcoholism
When all traditional therapeutic approaches fail for alcohol abuse disorder, a radical gene therapy might be something to try in the future.
Story by Freethink
A single shot — a gene therapy injected into the brain — dramatically reduced alcohol consumption in monkeys that previously drank heavily. If the therapy is safe and effective in people, it might one day be a permanent treatment for alcoholism for people with no other options.
The challenge: Alcohol use disorder (AUD) means a person has trouble controlling their alcohol consumption, even when it is negatively affecting their life, job, or health.
In the U.S., more than 10 percent of people over the age of 12 are estimated to have AUD, and while medications, counseling, or sheer willpower can help some stop drinking, staying sober can be a huge struggle — an estimated 40-60 percent of people relapse at least once.
A team of U.S. researchers suspected that an in-development gene therapy for Parkinson’s disease might work as a dopamine-replenishing treatment for alcoholism, too.
According to the CDC, more than 140,000 Americans are dying each year from alcohol-related causes, and the rate of deaths has been rising for years, especially during the pandemic.
The idea: For occasional drinkers, alcohol causes the brain to release more dopamine, a chemical that makes you feel good. Chronic alcohol use, however, causes the brain to produce, and process, less dopamine, and this persistent dopamine deficit has been linked to alcohol relapse.
There is currently no way to reverse the changes in the brain brought about by AUD, but a team of U.S. researchers suspected that an in-development gene therapy for Parkinson’s disease might work as a dopamine-replenishing treatment for alcoholism, too.
To find out, they tested it in heavy-drinking monkeys — and the animals’ alcohol consumption dropped by 90% over the course of a year.
How it works: The treatment centers on the protein GDNF (“glial cell line-derived neurotrophic factor”), which supports the survival of certain neurons, including ones linked to dopamine.
For the new study, a harmless virus was used to deliver the gene that codes for GDNF into the brains of four monkeys that, when they had the option, drank heavily — the amount of ethanol-infused water they consumed would be equivalent to a person having nine drinks per day.
“We targeted the cell bodies that produce dopamine with this gene to increase dopamine synthesis, thereby replenishing or restoring what chronic drinking has taken away,” said co-lead researcher Kathleen Grant.
To serve as controls, another four heavy-drinking monkeys underwent the same procedure, but with a saline solution delivered instead of the gene therapy.
The results: All of the monkeys had their access to alcohol removed for two months following the surgery. When it was then reintroduced for four weeks, the heavy drinkers consumed 50 percent less compared to the control group.
When the researchers examined the monkeys’ brains at the end of the study, they were able to confirm that dopamine levels had been replenished in the treated animals, but remained low in the controls.
The researchers then took the alcohol away for another four weeks, before giving it back for four. They repeated this cycle for a year, and by the end of it, the treated monkeys’ consumption had fallen by more than 90 percent compared to the controls.
“Drinking went down to almost zero,” said Grant. “For months on end, these animals would choose to drink water and just avoid drinking alcohol altogether. They decreased their drinking to the point that it was so low we didn’t record a blood-alcohol level.”
When the researchers examined the monkeys’ brains at the end of the study, they were able to confirm that dopamine levels had been replenished in the treated animals, but remained low in the controls.
Looking ahead: Dopamine is involved in a lot more than addiction, so more research is needed to not only see if the results translate to people but whether the gene therapy leads to any unwanted changes to mood or behavior.
Because the therapy requires invasive brain surgery and is likely irreversible, it’s unlikely to ever become a common treatment for alcoholism — but it could one day be the only thing standing between people with severe AUD and death.
“[The treatment] would be most appropriate for people who have already shown that all our normal therapeutic approaches do not work for them,” said Grant. “They are likely to create severe harm or kill themselves or others due to their drinking.”
This article originally appeared on Freethink, home of the brightest minds and biggest ideas of all time.


