Anti-Aging Pioneer Aubrey de Grey: “People in Middle Age Now Have a Fair Chance”
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

Aubrey de Grey at the World Stem Cell Summit in Miami on January 25, 2018.
Aging is not a mystery, says famed researcher Dr. Aubrey de Grey, perhaps the world's foremost advocate of the provocative view that medical technology will one day allow humans to control the aging process and live healthily into our hundreds—or even thousands.
"The cultural attitudes toward all of this are going to be completely turned upside down by sufficiently promising results in the lab, in mice."
He likens aging to a car wearing down over time; as the body operates normally, it accumulates damage which can be tolerated for a while, but eventually sends us into steep decline. The most promising way to escape this biological reality, he says, is to repair the damage as needed with precise scientific tools.
The bad news is that doing this groundbreaking research takes a long time and a lot of money, which has not always been readily available, in part due to a cultural phenomenon he terms "the pro-aging trance." Cultural attitudes have long been fatalistic about the inevitability of aging; many people balk at the seemingly implausible prospect of indefinite longevity.
But the good news for de Grey—and those who are cheering him on—is that his view is becoming less radical these days. Both the academic and private sectors are racing to tackle aging; his own SENS Research Foundation, for one, has spun out into five different companies. Defeating aging, he says, "is not just a future industry; it's an industry now that will be both profitable and extremely good for your health."
De Grey sat down with Editor-in-Chief Kira Peikoff at the World Stem Cell Summit in Miami to give LeapsMag the latest scoop on his work. Here is an edited and condensed version of our conversation.
Since your book Ending Aging was published a decade ago, scientific breakthroughs in stem cell research, genome editing, and other fields have taken the world by storm. Which of these have most affected your research?
They have all affected it a lot in one way, and hardly at all in another way. They have speeded it up--facilitated short cuts, ways to get where we're already trying to go. What they have not done is identified any fundamental changes to the overall strategy. In the book, we described the seven major types of damage, and particular ways of going about fixing each of them, and that hasn't changed.
"Repair at the microscopic level, one would be able to expect to do without surgery, just by injecting the right kind of stem cells."
Has any breakthrough specifically made the biggest impact?
It's not just the obvious things, like iPS (induced pluripotent stem cells) and CRISPR (a precise tool for editing genes). It's also the more esoteric things that applied specifically to certain of our areas, but most people don't really know about them. For example, the identification of how to control something called co-translational mitochondrial protein import.
How much of the future of anti-aging treatments will involve regeneration of old tissue, or wholesale growth of new organs?
The more large-scale ones, regenerating whole new organs, are probably only going to play a role in the short-term and will be phased out relatively rapidly, simply because, in order to be useful, one has to employ surgery, which is really invasive. We'll want to try to get around that, but it seems quite likely that in the very early stages, the techniques we have for repairing things at the molecular and cellular level in situ will be insufficiently comprehensive, and so we will need to do the more sledgehammer approach of building a whole new organ and sticking it in.
Every time you are in a position where you're replacing an organ, you have the option, in principle, of repairing the organ, without replacing it. And repair at the microscopic level, one would be able to expect to do without surgery, just by injecting the right kind of stem cells or whatever. That would be something one would expect to be able to apply to someone much closer to death's door and much more safely in general, and probably much more cheaply. One would expect that subsequent generations of these therapies would move in that direction.
Your foundation is working on an initiative requiring $50 million in funding—
Well, if we had $50 million per year in funding, we could go about three times faster than we are on $5 million per year.
And you're looking at a 2021 timeframe to start human trials?
That's approximate. Remember, because we accumulate in the body so many different types of damage, that means we have many different types of therapy to repair that damage. And of course, each of those types has to be developed independently. It's very much a divide and conquer therapy. The therapies interact with each other to some extent; the repair of one type of damage may slow down the creation of another type of damage, but still that's how it's going to be.
And some of these therapies are much easier to implement than others. The easier components of what we need to do are already in clinical trials—stem cell therapies especially, and immunotherapy against amyloid in the brain, for example. Even in phase III clinical trials in some cases. So when I talk about a timeframe like 2021, or early 20s shall we say, I'm really talking about the most difficult components.
What recent strides are you most excited about?
Looking back over the past couple of years, I'm particularly proud of the successes we've had in the very most difficult areas. If you go through the 7 components of SENS, there are two that have absolutely been stuck in a rut and have gotten nowhere for 15 to 20 years, and we basically fixed that in both cases. We published two years ago in Science magazine that essentially showed a way forward against the stiffening of the extracellular matrix, which is responsible for things like wrinkles and hypertension. And then a year ago, we published a real breakthrough paper with regard to placing copies of the mitochondria DNA in the nuclear DNA modified in such a way that they still work, which is an idea that had been around for 30 years; everyone had given up on it, some a long time ago, and we basically revived it.
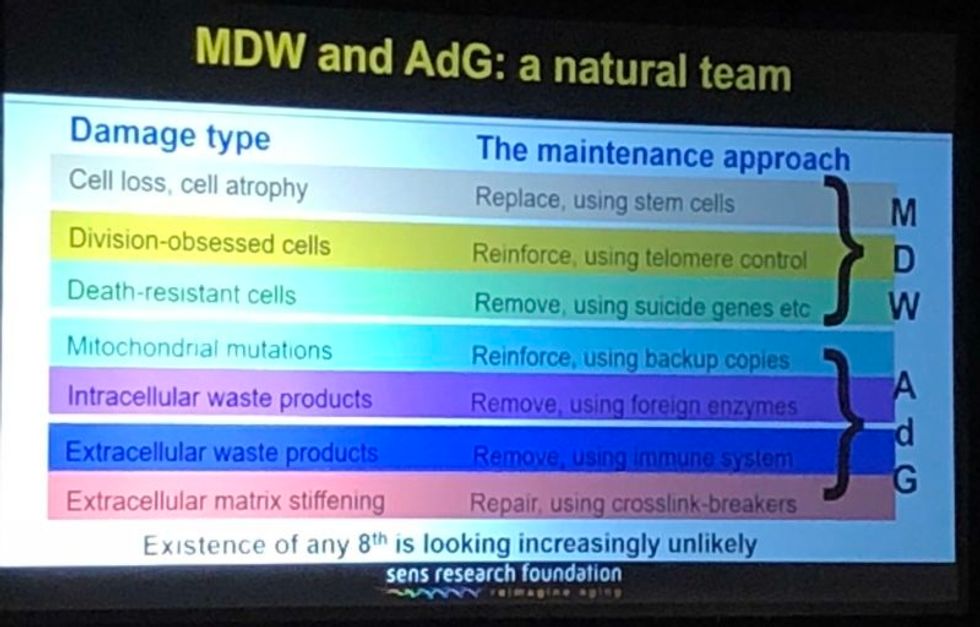
A slide presented by Aubrey de Grey, referencing his collaboration with Mike West at AgeX, showing the 7 types of damage that he believes must be repaired to end aging.
(Courtesy Kira Peikoff)
That's exciting. What do you think are the biggest barriers to defeating aging today: the technological challenges, the regulatory framework, the cost, or the cultural attitude of the "pro-aging" trance?
One can't really address those independently of each other. The technological side is one thing; it's hard, but we know where we're going, we've got a plan. The other ones are very intertwined with each other. A lot of people are inclined to say, the regulatory hurdle will be completely insurmountable, plus people don't recognize aging as a disease, so it's going to be a complete nonstarter. I think that's nonsense. And the reason is because the cultural attitudes toward all of this are going to be completely turned upside down before we have to worry about the regulatory hurdles. In other words, they're going to be turned upside down by sufficiently promising results in the lab, in mice. Once we get to be able to rejuvenate actually old mice really well so they live substantially longer than they otherwise would have done, in a healthy state, everyone's going to know about it and everyone's going to demand – it's not going to be possible to get re-elected unless you have a manifesto commitment to turn the FDA completely upside down and make sure this happens without any kind of regulatory obstacle.
I've been struggling away all these years trying to bring little bits of money in the door, and the reason I have is because of the skepticism as to regards whether this could actually work, combined with the pro-aging trance, which is a product of the skepticism – people not wanting to get their hopes up, so finding excuses about aging being a blessing in disguise, so they don't have to think about it. All of that will literally disintegrate pretty much overnight when we have the right kind of sufficiently impressive progress in the lab. Therefore, the availability of money will also [open up]. It's already cracking: we're already seeing the beginnings of the actual rejuvenation biotechnology industry that I've been talking about with a twinkle in my eye for some years.
"For humans, a 50-50 chance would be twenty years at this point, and there's a 10 percent chance that we won't get there for a hundred years."
Why do you think the culture is starting to shift?
There's no one thing yet. There will be that tipping point I mentioned, perhaps five years from now when we get a real breakthrough, decisive results in mice that make it simply impossible to carry on being fatalistic about all this. Prior to that, what we're already seeing is the impact of sheer old-school repeat advertising—me going out there, banging away and saying the same fucking thing again and again, and nobody saying anything that persuasively knocks me down. … And it's also the fact that we are making incremental amounts of progress, not just ourselves, but the scientific community generally. It has become incrementally more plausible that what I say might be true.
I'm sure you hate getting the timeline question, but if we're five years away from this breakthrough in mice, it's hard to resist asking—how far is that in terms of a human cure?
When I give any kind of timeframes, the only real care I have to take is to emphasize the variance. In this case I think we have got a 50-50 chance of getting to that tipping point in mice within five years from now, certainly it could be 10 or 15 years if we get unlucky. Similarly, for humans, a 50-50 chance would be twenty years at this point, and there's a 10 percent chance that we won't get there for a hundred years.
"I don't get people coming to me saying, well I don't think medicine for the elderly should be done because if it worked it would be a bad thing. People like to ignore this contradiction."
What would you tell skeptical people are the biggest benefits of a very long-lived population?
Any question about the longevity of people is the wrong question. Because the longevity that people fixate about so much will only ever occur as a side effect of health. However long ago you were born or however recently, if you're sick, you're likely to die fairly soon unless we can stop you being sick. Whereas if you're healthy, you're not. So if we do as well as we think we can do in terms of keeping people healthy and youthful however long ago they were born, then the side effect in terms of longevity and life expectancy is likely to be very large. But it's still a side effect, so the way that people actually ought to be—in fact have a requirement to be—thinking, is about whether they want people to be healthy.
Now I don't get people coming to me saying, well I don't think medicine for the elderly should be done because if it worked it would be a bad thing. People like to ignore this contradiction, they like to sweep it under the carpet and say, oh yeah, aging is totally a good thing.
People will never actually admit to the fact that what they are fundamentally saying is medicine for the elderly, if it actually works, would be bad, but still that is what they are saying.
Shifting gears a bit, I'm curious to find out which other radical visionaries in science and tech today you most admire?
Fair question. One is Mike West. I have the great privilege that I now work for him part-time with Age X. I have looked up to him very much for the past ten years, because what he did over the past 20 years starting with Geron is unimaginable today. He was working in an environment where I would not have dreamt of the possibility of getting any private money, any actual investment, in something that far out, that far ahead of its time, and he did it, again and again. It's insane what he managed to do.
What about someone like Elon Musk?
Sure, he's another one. He is totally impervious to the caution and criticism and conservatism that pervades humanity, and he's getting on making these bloody self-driving cars, space tourism, and so on, making them happen. He's thinking just the way I'm thinking really.
"You can just choose how frequently and how thoroughly you repair the damage. And you can make a different choice next time."
You famously said ten years ago that you think the first person to live to 1000 is already alive. Do you think that's still the case?
Definitely, yeah. I can't see how it could not be. Again, it's a probabilistic thing. I said there's at least a 10 percent chance that we won't get to what I call Longevity Escape Velocity for 100 years and if that's true, then the statement about 1000 years being alive already is not going to be the case. But for sure, I believe that the beneficiaries of what we may as well call SENS 1.0, the point where we get to LEV, those people are exceptionally unlikely ever to suffer from any kind of ill health correlated with their age. Because we will never fall below Longevity Escape Velocity once we attain it.
Could someone who was just born today expect—
I would say people in middle age now have a fair chance. Remember – a 50/50 chance of getting to LEV within 20 years, and when you get there, you don't just stay at biologically 70 or 80, you are rejuvenated back to biologically 30 or 40 and you stay there, so your risk of death each year is not related to how long ago you were born, it's the same as a young adult. Today, that's less than 1 in 1000 per year, and that number is going to go down as we get self-driving cars and all that, so actually 1000 is a very conservative number.
So you would be able to choose what age you wanted to go back to?
Oh sure, of course, it's just like a car. What you're doing is you're repairing damage, and the damage is still being created by the body's metabolism, so you can just choose how frequently and how thoroughly you repair the damage. And you can make a different choice next time.
What would be your perfect age?
I have no idea. That's something I don't have an opinion about, because I could change it whenever I like.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Chicken that is grown entirely in a laboratory, without harming a single bird, could be sold in supermarkets in the coming months. But critics say the doubts about lab-grown meat have not been appropriately explored.
Last November, when the U.S. Food and Drug Administration disclosed that chicken from a California firm called UPSIDE Foods did not raise safety concerns, it drily upended how humans have obtained animal protein for thousands of generations.
“The FDA is ready to work with additional firms developing cultured animal cell food and production processes to ensure their food is safe and lawful,” the agency said in a statement at the time.
Assuming UPSIDE obtains clearances from the U.S. Department of Agriculture, its chicken – grown entirely in a laboratory without harming a single bird – could be sold in supermarkets in the coming months.
“Ultimately, we want our products to be available everywhere meat is sold, including retail and food service channels,” a company spokesperson said. The upscale French restaurant Atelier Crenn in San Francisco will have UPSIDE chicken on its menu once it is approved, she added.
Known as lab-grown or cultured meat, a product such as UPSIDE’s is created using stem cells and other tissue obtained from a chicken, cow or other livestock. Those cells are then multiplied in a nutrient-dense environment, usually in conjunction with a “scaffold” of plant-based materials or gelatin to give them a familiar form, such as a chicken breast or a ribeye steak. A Dutch company called Mosa Meat claims it can produce 80,000 hamburgers derived from a cluster of tissue the size of a sesame seed.
Critics say the doubts about lab-grown meat and the possibility it could merge “Brave New World” with “The Jungle” and “Soylent Green” have not been appropriately explored.
That’s a far cry from when it took months of work to create the first lab-grown hamburger a decade ago. That minuscule patty – which did not contain any fat and was literally plucked from a Petri dish to go into a frying pan – cost about $325,000 to produce.
Just a decade later, an Israeli company called Future Meat said it can produce lab-grown meat for about $1.70 per pound. It plans to open a production facility in the U.S. sometime in 2023 and distribute its products under the brand name “Believer.”
Costs for production have sunk so low that researchers at Carnegie Mellon University in Pittsburgh expect sometime in early 2024 to produce lab-grown Wagyu steak to showcase the viability of growing high-end cuts of beef cheaply. The Carnegie Mellon team is producing its Wagyu using a consumer 3-D printer bought secondhand on eBay and modified to print the highly marbled flesh using a method developed by the university. The device costs $200 – about the same as a pound of Wagyu in the U.S. The initiative’s modest five-figure budget was successfully crowdfunded last year.
“The big cost is going to be the cells (which are being extracted by a cow somewhere in Pennsylvania), but otherwise printing doesn’t add much to the process,” said Rosalyn Abbott, a Carnegie Mellon assistant professor of bioengineering who is co-leader on the project. “But it adds value, unlike doing this with ground meat.”
Lab-Grown Meat’s Promise
Proponents of lab-grown meat say it will cut down on traditional agriculture, which has been a leading contributor to deforestation, water shortages and contaminated waterways from animal waste, as well as climate change.
An Oxford University study from 2011 concludes lab-grown meat could have greenhouse emissions 96 percent lower compared to traditionally raised livestock. Moreover, proponents of lab-grown meat claim that the suffering of animals would decline dramatically, as they would no longer need to be warehoused and slaughtered. A recently opened 26-story high-rise in China dedicated to the raising and slaughtering of pigs illustrates the current plight of livestock in stark terms.
Scientists may even learn how to tweak lab-grown meat to make it more nutritious. Natural red meat is high in saturated fat and, if it’s eaten too often, can lead to chronic diseases. In lab versions, the saturated fat could be swapped for healthier, omega-3 fatty acids.
But critics say the doubts about lab-grown meat and the possibility it could merge “Brave New World” with “The Jungle” and “Soylent Green” have not been appropriately explored.
A Slippery Slope?
Some academics who have studied the moral and ethical issues surrounding lab-grown meat believe it will have a tough path ahead gaining acceptance by consumers. Should it actually succeed in gaining acceptance, many ethical questions must be answered.
“People might be interested” in lab-grown meat, perhaps as a curiosity, said Carlos Alvaro, an associate professor of philosophy at the New York City College of Technology, part of the City University of New York. But the allure of traditionally sourced meat has been baked – or perhaps grilled – into people’s minds for so long that they may not want to make the switch. Plant-based meat provides a recent example of the uphill battle involved in changing old food habits, with Beyond Meat’s stock prices dipping nearly 80 percent in 2022.
"There are many studies showing that people don’t really care about the environment (to that extent)," Alvaro said. "So I don’t know how you would convince people to do this because of the environment.”
“From my research, I understand that the taste (of lab-grown meat) is not quite there,” Alvaro said, noting that the amino acids, sugars and other nutrients required to grow cultivated meat do not mimic what livestock are fed. He also observed that the multiplication of cells as part of the process “really mimic cancer cells” in the way they grow, another off-putting thought for would-be consumers of the product.
Alvaro is also convinced the public will not buy into any argument that lab-grown meat is more environmentally friendly.
“If people care about the environment, they either try and consume considerably less meat and other animal products, or they go vegan or vegetarian,” he said. “But there are many studies showing that people don’t really care about the environment (to that extent). So I don’t know how you would convince people to do this because of the environment.”
Ben Bramble, a professor at Australian National University who previously held posts at Princeton and Trinity College in Ireland, takes a slightly different tack. He noted that “if lab-grown meat becomes cheaper, healthier, or tastier than regular meat, there will be a large market for it. If it becomes all of these things, it will dominate the market.”
However, Bramble has misgivings about that occurring. He believes a smooth transition from traditionally sourced meat to a lab-grown version would allow humans to elide over the decades of animal cruelty perpetrated by large-scale agriculture, without fully reckoning with and learning from this injustice.
“My fear is that if we all switch over to lab-grown meat because it has become cheaper, healthier, or tastier than regular meat, we might never come to realize what we have done, and the terrible things we are capable of,” he said. “This would be a catastrophe.”
Bramble’s writings about cultured meat also raise some serious moral conundrums. If, for example, animal meat may be cultivated without killing animals, why not create products from human protein?
Actually, that’s already happened.
It occurred in 2019, when Orkan Telhan, a professor of fine arts at the University of Pennsylvania, collaborated with two scientists to create an art exhibit at the Philadelphia Museum of Art on the future of foodstuffs.
Although the exhibit included bioengineered bread and genetically modified salmon, it was an installation called “Ouroboros Steak” that drew the most attention. That was comprised of pieces of human flesh grown in a lab from cultivated cells and expired blood products obtained from online sources.
The exhibit was presented as four tiny morsels of red meat – shaped in patterns suggesting an ouroboros, a dragon eating its own tail. They were placed in tiny individual saucers atop a larger plate and placemat with a calico pattern, suggesting an item to order in a diner. The artwork drew international headlines – as well as condemnation for Telhan’s vision.
Telhan’s artwork is intended to critique the overarching assumption that lab-grown meat will eventually replace more traditional production methods, as well as the lack of transparency surrounding many processed foodstuffs. “They think that this problem (from industrial-scale agriculture) is going be solved by this new technology,” Telhan said. “I am critical (of) that perspective.”
Unlike Bramble, Telhan is not against lab-grown meat, so long as its producers are transparent about the sourcing of materials and its cultivation. But he believes that large-scale agricultural meat production – which dates back centuries – is not going to be replaced so quickly.
“We see this again and again with different industries, like algae-based fuels. A lot of companies were excited about this, and promoted it,” Telhan said. “And years later, we know these fuels work. But to be able to displace the oil industry means building the infrastructure to scale takes billions of dollars, and nobody has the patience or money to do it.”
Alvaro concurred on this point, which he believes is already weakened because a large swath of consumers aren’t concerned about environmental degradation.
“They’re going to have to sell this big, but in order to convince people to do so, they have to convince them to eat this product instead of regular meat,” Alvaro said.
Hidden Tweaks?
Moreover, if lab-based meat does obtain a significant market share, Telhan suggested companies may do things to the product – such as to genetically modify it to become more profitable – and never notify consumers. That is a particular concern in the U.S., where regulations regarding such modifications are vastly more relaxed than in the European Union.
“I think that they have really good objectives, and they aspire to good objectives,” Telhan said. “But the system itself doesn't really allow for that much transparency.”
No matter what the future holds, sometime next year Carnegie Mellon is expected to hold a press conference announcing it has produced a cut of the world’s most expensive beef with the help of a modified piece of consumer electronics. It will likely take place at around the same time UPSIDE chicken will be available for purchase in supermarkets and restaurants, pending the USDA’s approvals.
Abbott, the Carnegie Mellon professor, suggested the future event will be both informative and celebratory.
“I think Carnegie Mellon would have someone potentially cook it for us,” she said. “Like have a really good chef in New York City do it.”
In this week's Friday Five, breathing this way may cut down on anxiety, a fasting regimen that could make you sick, this type of job makes men more virile, 3D printed hearts could save your life, and the role of metformin in preventing dementia.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Here are the promising studies covered in this week's Friday Five, featuring interviews with Dr. David Spiegel, associate chair of psychiatry and behavioral sciences at Stanford, and Dr. Filip Swirski, professor of medicine and cardiology at the Icahn School of Medicine at Mount Sinai.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Here are the promising studies covered in this week's Friday Five, featuring interviews with Dr. David Spiegel, associate chair of psychiatry and behavioral sciences at Stanford, and Dr. Filip Swirski, professor of medicine and cardiology at the Icahn School of Medicine at Mount Sinai.
- Breathing this way cuts down on anxiety*
- Could your fasting regimen make you sick?
- This type of job makes men more virile
- 3D printed hearts could save your life
- Yet another potential benefit of metformin
* This video with Dr. Andrew Huberman of Stanford shows exactly how to do the breathing practice.