Anti-Aging Pioneer Aubrey de Grey: “People in Middle Age Now Have a Fair Chance”
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

Aubrey de Grey at the World Stem Cell Summit in Miami on January 25, 2018.
Aging is not a mystery, says famed researcher Dr. Aubrey de Grey, perhaps the world's foremost advocate of the provocative view that medical technology will one day allow humans to control the aging process and live healthily into our hundreds—or even thousands.
"The cultural attitudes toward all of this are going to be completely turned upside down by sufficiently promising results in the lab, in mice."
He likens aging to a car wearing down over time; as the body operates normally, it accumulates damage which can be tolerated for a while, but eventually sends us into steep decline. The most promising way to escape this biological reality, he says, is to repair the damage as needed with precise scientific tools.
The bad news is that doing this groundbreaking research takes a long time and a lot of money, which has not always been readily available, in part due to a cultural phenomenon he terms "the pro-aging trance." Cultural attitudes have long been fatalistic about the inevitability of aging; many people balk at the seemingly implausible prospect of indefinite longevity.
But the good news for de Grey—and those who are cheering him on—is that his view is becoming less radical these days. Both the academic and private sectors are racing to tackle aging; his own SENS Research Foundation, for one, has spun out into five different companies. Defeating aging, he says, "is not just a future industry; it's an industry now that will be both profitable and extremely good for your health."
De Grey sat down with Editor-in-Chief Kira Peikoff at the World Stem Cell Summit in Miami to give LeapsMag the latest scoop on his work. Here is an edited and condensed version of our conversation.
Since your book Ending Aging was published a decade ago, scientific breakthroughs in stem cell research, genome editing, and other fields have taken the world by storm. Which of these have most affected your research?
They have all affected it a lot in one way, and hardly at all in another way. They have speeded it up--facilitated short cuts, ways to get where we're already trying to go. What they have not done is identified any fundamental changes to the overall strategy. In the book, we described the seven major types of damage, and particular ways of going about fixing each of them, and that hasn't changed.
"Repair at the microscopic level, one would be able to expect to do without surgery, just by injecting the right kind of stem cells."
Has any breakthrough specifically made the biggest impact?
It's not just the obvious things, like iPS (induced pluripotent stem cells) and CRISPR (a precise tool for editing genes). It's also the more esoteric things that applied specifically to certain of our areas, but most people don't really know about them. For example, the identification of how to control something called co-translational mitochondrial protein import.
How much of the future of anti-aging treatments will involve regeneration of old tissue, or wholesale growth of new organs?
The more large-scale ones, regenerating whole new organs, are probably only going to play a role in the short-term and will be phased out relatively rapidly, simply because, in order to be useful, one has to employ surgery, which is really invasive. We'll want to try to get around that, but it seems quite likely that in the very early stages, the techniques we have for repairing things at the molecular and cellular level in situ will be insufficiently comprehensive, and so we will need to do the more sledgehammer approach of building a whole new organ and sticking it in.
Every time you are in a position where you're replacing an organ, you have the option, in principle, of repairing the organ, without replacing it. And repair at the microscopic level, one would be able to expect to do without surgery, just by injecting the right kind of stem cells or whatever. That would be something one would expect to be able to apply to someone much closer to death's door and much more safely in general, and probably much more cheaply. One would expect that subsequent generations of these therapies would move in that direction.
Your foundation is working on an initiative requiring $50 million in funding—
Well, if we had $50 million per year in funding, we could go about three times faster than we are on $5 million per year.
And you're looking at a 2021 timeframe to start human trials?
That's approximate. Remember, because we accumulate in the body so many different types of damage, that means we have many different types of therapy to repair that damage. And of course, each of those types has to be developed independently. It's very much a divide and conquer therapy. The therapies interact with each other to some extent; the repair of one type of damage may slow down the creation of another type of damage, but still that's how it's going to be.
And some of these therapies are much easier to implement than others. The easier components of what we need to do are already in clinical trials—stem cell therapies especially, and immunotherapy against amyloid in the brain, for example. Even in phase III clinical trials in some cases. So when I talk about a timeframe like 2021, or early 20s shall we say, I'm really talking about the most difficult components.
What recent strides are you most excited about?
Looking back over the past couple of years, I'm particularly proud of the successes we've had in the very most difficult areas. If you go through the 7 components of SENS, there are two that have absolutely been stuck in a rut and have gotten nowhere for 15 to 20 years, and we basically fixed that in both cases. We published two years ago in Science magazine that essentially showed a way forward against the stiffening of the extracellular matrix, which is responsible for things like wrinkles and hypertension. And then a year ago, we published a real breakthrough paper with regard to placing copies of the mitochondria DNA in the nuclear DNA modified in such a way that they still work, which is an idea that had been around for 30 years; everyone had given up on it, some a long time ago, and we basically revived it.
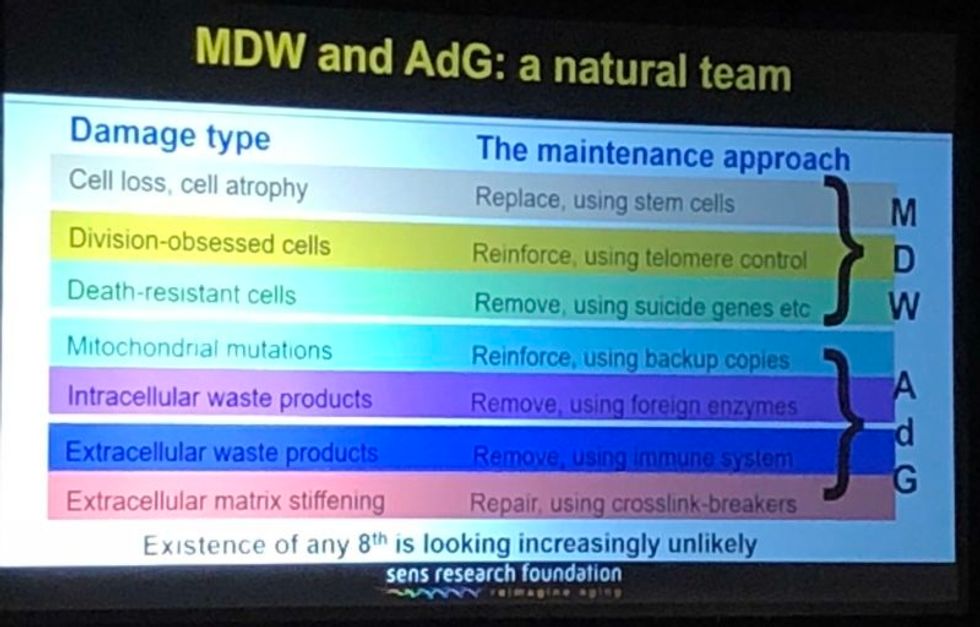
A slide presented by Aubrey de Grey, referencing his collaboration with Mike West at AgeX, showing the 7 types of damage that he believes must be repaired to end aging.
(Courtesy Kira Peikoff)
That's exciting. What do you think are the biggest barriers to defeating aging today: the technological challenges, the regulatory framework, the cost, or the cultural attitude of the "pro-aging" trance?
One can't really address those independently of each other. The technological side is one thing; it's hard, but we know where we're going, we've got a plan. The other ones are very intertwined with each other. A lot of people are inclined to say, the regulatory hurdle will be completely insurmountable, plus people don't recognize aging as a disease, so it's going to be a complete nonstarter. I think that's nonsense. And the reason is because the cultural attitudes toward all of this are going to be completely turned upside down before we have to worry about the regulatory hurdles. In other words, they're going to be turned upside down by sufficiently promising results in the lab, in mice. Once we get to be able to rejuvenate actually old mice really well so they live substantially longer than they otherwise would have done, in a healthy state, everyone's going to know about it and everyone's going to demand – it's not going to be possible to get re-elected unless you have a manifesto commitment to turn the FDA completely upside down and make sure this happens without any kind of regulatory obstacle.
I've been struggling away all these years trying to bring little bits of money in the door, and the reason I have is because of the skepticism as to regards whether this could actually work, combined with the pro-aging trance, which is a product of the skepticism – people not wanting to get their hopes up, so finding excuses about aging being a blessing in disguise, so they don't have to think about it. All of that will literally disintegrate pretty much overnight when we have the right kind of sufficiently impressive progress in the lab. Therefore, the availability of money will also [open up]. It's already cracking: we're already seeing the beginnings of the actual rejuvenation biotechnology industry that I've been talking about with a twinkle in my eye for some years.
"For humans, a 50-50 chance would be twenty years at this point, and there's a 10 percent chance that we won't get there for a hundred years."
Why do you think the culture is starting to shift?
There's no one thing yet. There will be that tipping point I mentioned, perhaps five years from now when we get a real breakthrough, decisive results in mice that make it simply impossible to carry on being fatalistic about all this. Prior to that, what we're already seeing is the impact of sheer old-school repeat advertising—me going out there, banging away and saying the same fucking thing again and again, and nobody saying anything that persuasively knocks me down. … And it's also the fact that we are making incremental amounts of progress, not just ourselves, but the scientific community generally. It has become incrementally more plausible that what I say might be true.
I'm sure you hate getting the timeline question, but if we're five years away from this breakthrough in mice, it's hard to resist asking—how far is that in terms of a human cure?
When I give any kind of timeframes, the only real care I have to take is to emphasize the variance. In this case I think we have got a 50-50 chance of getting to that tipping point in mice within five years from now, certainly it could be 10 or 15 years if we get unlucky. Similarly, for humans, a 50-50 chance would be twenty years at this point, and there's a 10 percent chance that we won't get there for a hundred years.
"I don't get people coming to me saying, well I don't think medicine for the elderly should be done because if it worked it would be a bad thing. People like to ignore this contradiction."
What would you tell skeptical people are the biggest benefits of a very long-lived population?
Any question about the longevity of people is the wrong question. Because the longevity that people fixate about so much will only ever occur as a side effect of health. However long ago you were born or however recently, if you're sick, you're likely to die fairly soon unless we can stop you being sick. Whereas if you're healthy, you're not. So if we do as well as we think we can do in terms of keeping people healthy and youthful however long ago they were born, then the side effect in terms of longevity and life expectancy is likely to be very large. But it's still a side effect, so the way that people actually ought to be—in fact have a requirement to be—thinking, is about whether they want people to be healthy.
Now I don't get people coming to me saying, well I don't think medicine for the elderly should be done because if it worked it would be a bad thing. People like to ignore this contradiction, they like to sweep it under the carpet and say, oh yeah, aging is totally a good thing.
People will never actually admit to the fact that what they are fundamentally saying is medicine for the elderly, if it actually works, would be bad, but still that is what they are saying.
Shifting gears a bit, I'm curious to find out which other radical visionaries in science and tech today you most admire?
Fair question. One is Mike West. I have the great privilege that I now work for him part-time with Age X. I have looked up to him very much for the past ten years, because what he did over the past 20 years starting with Geron is unimaginable today. He was working in an environment where I would not have dreamt of the possibility of getting any private money, any actual investment, in something that far out, that far ahead of its time, and he did it, again and again. It's insane what he managed to do.
What about someone like Elon Musk?
Sure, he's another one. He is totally impervious to the caution and criticism and conservatism that pervades humanity, and he's getting on making these bloody self-driving cars, space tourism, and so on, making them happen. He's thinking just the way I'm thinking really.
"You can just choose how frequently and how thoroughly you repair the damage. And you can make a different choice next time."
You famously said ten years ago that you think the first person to live to 1000 is already alive. Do you think that's still the case?
Definitely, yeah. I can't see how it could not be. Again, it's a probabilistic thing. I said there's at least a 10 percent chance that we won't get to what I call Longevity Escape Velocity for 100 years and if that's true, then the statement about 1000 years being alive already is not going to be the case. But for sure, I believe that the beneficiaries of what we may as well call SENS 1.0, the point where we get to LEV, those people are exceptionally unlikely ever to suffer from any kind of ill health correlated with their age. Because we will never fall below Longevity Escape Velocity once we attain it.
Could someone who was just born today expect—
I would say people in middle age now have a fair chance. Remember – a 50/50 chance of getting to LEV within 20 years, and when you get there, you don't just stay at biologically 70 or 80, you are rejuvenated back to biologically 30 or 40 and you stay there, so your risk of death each year is not related to how long ago you were born, it's the same as a young adult. Today, that's less than 1 in 1000 per year, and that number is going to go down as we get self-driving cars and all that, so actually 1000 is a very conservative number.
So you would be able to choose what age you wanted to go back to?
Oh sure, of course, it's just like a car. What you're doing is you're repairing damage, and the damage is still being created by the body's metabolism, so you can just choose how frequently and how thoroughly you repair the damage. And you can make a different choice next time.
What would be your perfect age?
I have no idea. That's something I don't have an opinion about, because I could change it whenever I like.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Staying well in the 21st century is like playing a game of chess
The control of infectious diseases was considered to be one of the “10 Great Public Health Achievements.” What we didn’t take into account was the very concept of evolution: as we built better protections, our enemies eventually boosted their attacking prowess, so soon enough we found ourselves on the defensive once again.
This article originally appeared in One Health/One Planet, a single-issue magazine that explores how climate change and other environmental shifts are increasing vulnerabilities to infectious diseases by land and by sea. The magazine probes how scientists are making progress with leaders in other fields toward solutions that embrace diverse perspectives and the interconnectedness of all lifeforms and the planet.
On July 30, 1999, the Centers for Disease Control and Prevention published a report comparing data on the control of infectious disease from the beginning of the 20th century to the end. The data showed that deaths from infectious diseases declined markedly. In the early 1900s, pneumonia, tuberculosis and diarrheal diseases were the three leading killers, accounting for one-third of total deaths in the U.S.—with 40 percent being children under five.
Mass vaccinations, the discovery of antibiotics and overall sanitation and hygiene measures eventually eradicated smallpox, beat down polio, cured cholera, nearly rid the world of tuberculosis and extended the U.S. life expectancy by 25 years. By 1997, there was a shift in population health in the U.S. such that cancer, diabetes and heart disease were now the leading causes of death.
The control of infectious diseases is considered to be one of the “10 Great Public Health Achievements.” Yet on the brink of the 21st century, new trouble was already brewing. Hospitals were seeing periodic cases of antibiotic-resistant infections. Novel viruses, or those that previously didn’t afflict humans, began to emerge, causing outbreaks of West Nile, SARS, MERS or swine flu.In the years that followed, tuberculosis made a comeback, at least in certain parts of the world. What we didn’t take into account was the very concept of evolution: as we built better protections, our enemies eventually boosted their attacking prowess, so soon enough we found ourselves on the defensive once again.
At the same time, new, previously unknown or extremely rare disorders began to rise, such as autoimmune or genetic conditions. Two decades later, scientists began thinking about health differently—not as a static achievement guaranteed to last, but as something dynamic and constantly changing—and sometimes, for the worse.
What emerged since then is a different paradigm that makes our interactions with the microbial world more like a biological chess match, says Victoria McGovern, a biochemist and program officer for the Burroughs Wellcome Fund’s Infectious Disease and Population Sciences Program. In this chess game, humans may make a clever strategic move, which could involve creating a new vaccine or a potent antibiotic, but that advantage is fleeting. At some point, the organisms we are up against could respond with a move of their own—such as developing resistance to medication or genetic mutations that attack our bodies. Simply eradicating the “opponent,” or the pathogenic microbes, as efficiently as possible isn’t enough to keep humans healthy long-term.
Instead, scientists should focus on studying the complexity of interactions between humans and their pathogens. “We need to better understand the lifestyles of things that afflict us,” McGovern says. “The solutions are going to be in understanding various parts of their biology so we can influence how they behave around our systems.”
Genetics and cell biology, combined with imaging techniques that allow one to see tissues and individual cells in actions, will enable scientists to define and quantify what it means to be healthy at the molecular level.
What is being proposed will require a pivot to basic biology and other disciplines that have suffered from lack of research funding in recent years. Yet, according to McGovern, the research teams of funded proposals are answering bigger questions. “We look for people exploring questions about hosts and pathogens, and what happens when they touch, but we’re also looking for people with big ideas,” she says. For example, if one specific infection causes a chain of pathological events in the body, can other infections cause them too? And if we find a way to break that chain for one pathogen, can we play the same trick on another? “We really want to see people thinking of not just one experiment but about big implications of their work,” McGovern says.
Jonah Cool, a cell biologist, geneticist and science officer at the Chan Zuckerberg Initiative, says that it’s necessary to define what constitutes a healthy organism and how it overcomes infections or environmental assaults, such as pollution from forest fires or toxins from industrial smokestacks. An organism that catches a disease isn’t necessarily an unhealthy one, as long as it fights it off successfully—an ability that arises from the complex interplay of its genes, the immune system, age, stress levels and other factors. Modern science allows many of these factors to be measured, recorded and compared. “We need a data-driven, deep-phenotyping approach to defining healthy biological systems and their responses to insults—which can be infectious disease or environmental exposures—and their ability to navigate their way through that space,” Cool says.
Genetics and cell biology, combined with imaging techniques that allow one to see tissues and individual cells in actions, will enable scientists to define and quantify what it means to be healthy at the molecular level. “As a geneticist and cell biologist, I believe in all these molecular underpinnings and how they arise in phenotypic differences in cells, genes, proteins—and how their combinations form complex cellular states,” Cool says.
Julie Graves, a physician, public health consultant, former adjunct professor of management, policy and community health at the University of Texas Health Science Center in Houston, stresses the necessity of nutritious diets. According to the Rockefeller Food Initiative, “poor diet is the leading risk factor for disease, disability and premature death in the majority of countries around the world.” Adequate nutrition is critical for maintaining human health and life. Yet, Western diets are often low in essential nutrients, high in calories and heavy on processed foods. Overconsumption of these foods has contributed to high rates of obesity and chronic disease in the U.S. In fact, more than half of American adults have at least one chronic disease, and 27 percent have more than one—which increases vulnerability to COVID-19 infections, according to the 2018 National Health Interview Survey.
Further, the contamination of our food supply with various agricultural and industrial toxins—petrochemicals, pesticides, PFAS and others—has implications for morbidity, mortality, and overall quality of life. “These chemicals are insidiously in everything, including our bodies,” Graves says—and they are interfering with our normal biological functions. “We need to stop how we manufacture food,” she adds, and rid our sustenance of these contaminants.
According to the Humane Society of the United States, factory farms result in nearly 40 percent of emissions of methane. Concentrated animal feeding operations or CAFOs may serve as breeding grounds for pandemics, scientists warn, so humans should research better ways to raise and treat livestock. Diego Rose, a professor of food and nutrition policy at Tulane University School of Public Health & Tropical Medicine, and his colleagues found that “20 percent of Americans’ diets account for about 45 percent of the environmental impacts [that come from food].” A subsequent study explored the impacts of specific foods and found that substituting beef for chicken lowers an individual’s carbon footprint by nearly 50 percent, with water usage decreased by 30 percent. Notably, however, eating too much red meat has been associated with a variety of illnesses.
In some communities, the option to swap food types is limited or impossible. For example, “many populations live in relative food deserts where there’s not a local grocery store that has any fresh produce,” says Louis Muglia, the president and CEO of Burroughs Wellcome. Individuals in these communities suffer from an insufficient intake of beneficial macronutrients, and they’re “probably being exposed to phenols and other toxins that are in the packaging.” An equitable, sustainable and nutritious food supply will be vital to humanity’s wellbeing in the era of climate change, unpredictable weather and spillover events.
A recent report by See Change Institute and the Climate Mental Health Network showed that people who are experiencing socioeconomic inequalities, including many people of color, contribute the least to climate change, yet they are impacted the most. For example, people in low-income communities are disproportionately exposed to vehicle emissions, Muglia says. Through its Climate Change and Human Health Seed Grants program, Burroughs Wellcome funds research that aims to understand how various factors related to climate change and environmental chemicals contribute to premature births, associated with health vulnerabilities over the course of a person’s life—and map such hot spots.
“It’s very complex, the combinations of socio-economic environment, race, ethnicity and environmental exposure, whether that’s heat or toxic chemicals,” Muglia explains. “Disentangling those things really requires a very sophisticated, multidisciplinary team. That’s what we’ve put together to describe where these hotspots are and see how they correlate with different toxin exposure levels.”
In addition to mapping the risks, researchers are developing novel therapeutics that will be crucial to our armor arsenal, but we will have to be smarter at designing and using them. We will need more potent, better-working monoclonal antibodies. Instead of directly attacking a pathogen, we may have to learn to stimulate the immune system—training it to fight the disease-causing microbes on its own. And rather than indiscriminately killing all bacteria with broad-scope drugs, we would need more targeted medications. “Instead of wiping out the entire gut flora, we will need to come up with ways that kill harmful bacteria but not healthy ones,” Graves says. Training our immune systems to recognize and react to pathogens by way of vaccination will keep us ahead of our biological opponents, too. “Continued development of vaccines against infectious diseases is critical,” says Graves.
With all of the unpredictable events that lie ahead, it is difficult to foresee what achievements in public health will be reported at the end of the 21st century. Yet, technological advances, better modeling and pursuing bigger questions in science, along with education and working closely with communities will help overcome the challenges. The Chan Zuckerberg Initiative displays an optimistic message on its website: “Is it possible to cure, prevent, or manage all diseases by the end of this century? We think so.” Cool shares the view of his employer—and believes that science can get us there. Just give it some time and a chance. “It’s a big, bold statement,” he says, “but the end of the century is a long way away.”Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
Alzheimer’s prevention may be less about new drugs, more about income, zip code and education
(Left to right) Vickie Naylor, Bernadine Clay, and Donna Maxey read a memory prompt as they take part in the Sharing History through Active Reminiscence and Photo-Imagery (SHARP) study, September 20, 2017.
That your risk of Alzheimer’s disease depends on your salary, what you ate as a child, or the block where you live may seem implausible. But researchers are discovering that social determinants of health (SDOH) play an outsized role in Alzheimer’s disease and related dementias, possibly more than age, and new strategies are emerging for how to address these factors.
At the 2022 Alzheimer’s Association International Conference, a series of presentations offered evidence that a string of socioeconomic factors—such as employment status, social support networks, education and home ownership—significantly affected dementia risk, even when adjusting data for genetic risk. What’s more, memory declined more rapidly in people who earned lower wages and slower in people who had parents of higher socioeconomic status.
In 2020, a first-of-its kind study in JAMA linked Alzheimer’s incidence to “neighborhood disadvantage,” which is based on SDOH indicators. Through autopsies, researchers analyzed brain tissue markers related to Alzheimer’s and found an association with these indicators. In 2022, Ryan Powell, the lead author of that study, published further findings that neighborhood disadvantage was connected with having more neurofibrillary tangles and amyloid plaques, the main pathological features of Alzheimer's disease.
As of yet, little is known about the biological processes behind this, says Powell, director of data science at the Center for Health Disparities Research at the University of Wisconsin School of Medicine and Public Health. “We know the association but not the direct causal pathway.”
The corroborative findings keep coming. In a Nature study published a few months after Powell’s study, every social determinant investigated affected Alzheimer’s risk except for marital status. The links were highest for income, education, and occupational status.
Clinical trials on new Alzheimer’s medications get all the headlines but preventing dementia through policy and public health interventions should not be underestimated.
The potential for prevention is significant. One in three older adults dies with Alzheimer's or another dementia—more than breast and prostate cancers combined. Further, a 2020 report from the Lancet Commission determined that about 40 percent of dementia cases could theoretically be prevented or delayed by managing the risk factors that people can modify.
Take inactivity. Older adults who took 9,800 steps daily were half as likely to develop dementia over the next 7 years, in a 2022 JAMA study. Hearing loss, another risk factor that can be managed, accounts for about 9 percent of dementia cases.
Clinical trials on new Alzheimer’s medications get all the headlines but preventing dementia through policy and public health interventions should not be underestimated. Simply slowing the course of Alzheimer’s or delaying its onset by five years would cut the incidence in half, according to the Global Council on Brain Health.
Minorities Hit the Hardest
The World Health Organization defines SDOH as “conditions in which people are born, work, live, and age, and the wider set of forces and systems shaping the conditions of daily life.”
Anyone who exists on processed food, smokes cigarettes, or skimps on sleep has heightened risks for dementia. But minority groups get hit harder. Older Black Americans are twice as likely to have Alzheimer’s or another form of dementia as white Americans; older Hispanics are about one and a half times more likely.
This is due in part to higher rates of diabetes, obesity, and high blood pressure within these communities. These diseases are linked to Alzheimer’s, and SDOH factors multiply the risks. Blacks and Hispanics earn less income on average than white people. This means they are more likely to live in neighborhoods with limited access to healthy food, medical care, and good schools, and suffer greater exposure to noise (which impairs hearing) and air pollution—additional risk factors for dementia.
Related Reading: The Toxic Effects of Noise and What We're Not Doing About it
Plus, when Black people are diagnosed with dementia, their cognitive impairment and neuropsychiatric symptom are more advanced than in white patients. Why? Some African-Americans delay seeing a doctor because of perceived discrimination and a sense they will not be heard, says Carl V. Hill, chief diversity, equity, and inclusion officer at the Alzheimer’s Association.
Misinformation about dementia is another issue in Black communities. The thinking is that Alzheimer’s is genetic or age-related, not realizing that diet and physical activity can improve brain health, Hill says.
African Americans are severely underrepresented in clinical trials for Alzheimer’s, too. So, researchers miss the opportunity to learn more about health disparities. “It’s a bioethical issue,” Hill says. “The people most likely to have Alzheimer’s aren’t included in the trials.”
The Cure: Systemic Change
People think of lifestyle as a choice but there are limitations, says Muniza Anum Majoka, a geriatric psychiatrist and assistant professor of psychiatry at Yale University, who published an overview of SDOH factors that impact dementia. “For a lot of people, those choices [to improve brain health] are not available,” she says. If you don’t live in a safe neighborhood, for example, walking for exercise is not an option.
Hill wants to see the focus of prevention shift from individual behavior change to ensuring everyone has access to the same resources. Advice about healthy eating only goes so far if someone lives in a food desert. Systemic change also means increasing the number of minority physicians and recruiting minorities in clinical drug trials so studies will be relevant to these communities, Hill says.
Based on SDOH impact research, raising education levels has the most potential to prevent dementia. One theory is that highly educated people have a greater brain reserve that enables them to tolerate pathological changes in the brain, thus delaying dementia, says Majoka. Being curious, learning new things and problem-solving also contribute to brain health, she adds. Plus, having more education may be associated with higher socioeconomic status, more access to accurate information and healthier lifestyle choices.
New Strategies
The chasm between what researchers know about brain health and how the knowledge is being applied is huge. “There’s an explosion of interest in this area. We’re just in the first steps,” says Powell. One day, he predicts that physicians will manage Alzheimer’s through precision medicine customized to the patient’s specific risk factors and needs.
Raina Croff, assistant professor of neurology at Oregon Health & Science University School of Medicine, created the SHARP (Sharing History through Active Reminiscence and Photo-imagery) walking program to forestall memory loss in African Americans with mild cognitive impairment or early dementia.
Participants and their caregivers walk in historically black neighborhoods three times a week over six months. A smart tablet provides information about “Memory Markers” they pass, such as the route of a civil rights march. People celebrate their community and culture while “brain health is running in the background,” Croff says.

Photos and memory prompts engage participants in the SHARP program.
OHSU/Kristyna Wentz-Graff
The project began in 2015 as a pilot study in Croff’s hometown of Portland, Ore., expanded to Seattle, and will soon start in Oakland, Calif. “Walking is good for slowing [brain] decline,” she says. A post-study assessment of 40 participants in 2017 showed that half had higher cognitive scores after the program; 78 percent had lower blood pressure; and 44 percent lost weight. Those with mild cognitive impairment showed the most gains. The walkers also reported improved mood and energy along with increased involvement in other activities.
It’s never too late to reap the benefits of working your brain and being socially engaged, Majoka says.
In Milwaukee, the Wisconsin Alzheimer’s Institute launched the The Amazing Grace Chorus® to stave off cognitive decline in seniors. People in early stages of Alzheimer’s practice and perform six concerts each year. The activity provides opportunities for social engagement, mental stimulation, and a support network. Among the benefits, 55 percent reported better communication at home and nearly half of participants said they got involved with more activities after participating in the chorus.
Private companies are offering intervention services to healthcare providers and insurers to manage SDOH, too. One such service, MyHello, makes calls to at-risk people to assess their needs—be it food, transportation or simply a friendly voice. Having a social support network is critical for seniors, says Majoka, noting there was a steep decline in cognitive function among isolated elders during Covid lockdowns.
About 1 in 9 Americans age 65 or older live with Alzheimer’s today. With a surge in people with the disease predicted, public health professionals have to think more broadly about resource targets and effective intervention points, Powell says.
Beyond breakthrough pills, that is. Like Dorothy in Kansas discovering happiness was always in her own backyard, we are beginning to learn that preventing Alzheimer’s is in our reach if only we recognized it.

