Scientists redesign bacteria to tackle the antibiotic resistance crisis
Probiotic bacteria can be engineered to fight antibiotic-resistant superbugs by releasing chemicals that kill them.
In 1945, almost two decades after Alexander Fleming discovered penicillin, he warned that as antibiotics use grows, they may lose their efficiency. He was prescient—the first case of penicillin resistance was reported two years later. Back then, not many people paid attention to Fleming’s warning. After all, the “golden era” of the antibiotics age had just began. By the 1950s, three new antibiotics derived from soil bacteria — streptomycin, chloramphenicol, and tetracycline — could cure infectious diseases like tuberculosis, cholera, meningitis and typhoid fever, among others.
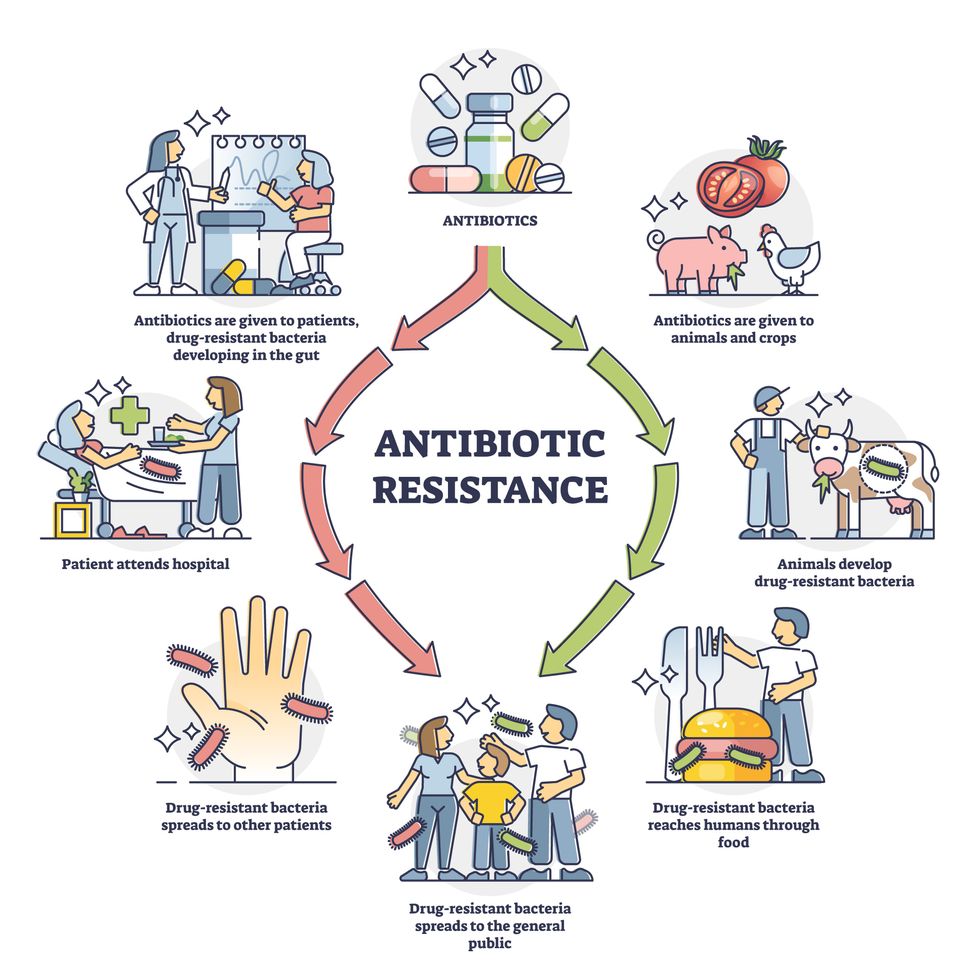
Today, these antibiotics and many of their successors developed through the 1980s are gradually losing their effectiveness. The extensive overuse and misuse of antibiotics led to the rise of drug resistance. The livestock sector buys around 80 percent of all antibiotics sold in the U.S. every year. Farmers feed cows and chickens low doses of antibiotics to prevent infections and fatten up the animals, which eventually causes resistant bacterial strains to evolve. If manure from cattle is used on fields, the soil and vegetables can get contaminated with antibiotic-resistant bacteria. Another major factor is doctors overprescribing antibiotics to humans, particularly in low-income countries. Between 2000 to 2018, the global rates of human antibiotic consumption shot up by 46 percent.
In recent years, researchers have been exploring a promising avenue: the use of synthetic biology to engineer new bacteria that may work better than antibiotics. The need continues to grow, as a Lancet study linked antibiotic resistance to over 1.27 million deaths worldwide in 2019, surpassing HIV/AIDS and malaria. The western sub-Saharan Africa region had the highest death rate (27.3 people per 100,000).
Researchers warn that if nothing changes, by 2050, antibiotic resistance could kill 10 million people annually.
To make it worse, our remedy pipelines are drying up. Out of the 18 biggest pharmaceutical companies, 15 abandoned antibiotic development by 2013. According to the AMR Action Fund, venture capital has remained indifferent towards biotech start-ups developing new antibiotics. In 2019, at least two antibiotic start-ups filed for bankruptcy. As of December 2020, there were 43 new antibiotics in clinical development. But because they are based on previously known molecules, scientists say they are inadequate for treating multidrug-resistant bacteria. Researchers warn that if nothing changes, by 2050, antibiotic resistance could kill 10 million people annually.
The rise of synthetic biology
To circumvent this dire future, scientists have been working on alternative solutions using synthetic biology tools, meaning genetically modifying good bacteria to fight the bad ones.
From the time life evolved on earth around 3.8 billion years ago, bacteria have engaged in biological warfare. They constantly strategize new methods to combat each other by synthesizing toxic proteins that kill competition.
For example, Escherichia coli produces bacteriocins or toxins to kill other strains of E.coli that attempt to colonize the same habitat. Microbes like E.coli (which are not all pathogenic) are also naturally present in the human microbiome. The human microbiome harbors up to 100 trillion symbiotic microbial cells. The majority of them are beneficial organisms residing in the gut at different compositions.
The chemicals that these “good bacteria” produce do not pose any health risks to us, but can be toxic to other bacteria, particularly to human pathogens. For the last three decades, scientists have been manipulating bacteria’s biological warfare tactics to our collective advantage.
In the late 1990s, researchers drew inspiration from electrical and computing engineering principles that involve constructing digital circuits to control devices. In certain ways, every cell in living organisms works like a tiny computer. The cell receives messages in the form of biochemical molecules that cling on to its surface. Those messages get processed within the cells through a series of complex molecular interactions.
Synthetic biologists can harness these living cells’ information processing skills and use them to construct genetic circuits that perform specific instructions—for example, secrete a toxin that kills pathogenic bacteria. “Any synthetic genetic circuit is merely a piece of information that hangs around in the bacteria’s cytoplasm,” explains José Rubén Morones-Ramírez, a professor at the Autonomous University of Nuevo León, Mexico. Then the ribosome, which synthesizes proteins in the cell, processes that new information, making the compounds scientists want bacteria to make. “The genetic circuit remains separated from the living cell’s DNA,” Morones-Ramírez explains. When the engineered bacteria replicates, the genetic circuit doesn’t become part of its genome.
Highly intelligent by bacterial standards, some multidrug resistant V. cholerae strains can also “collaborate” with other intestinal bacterial species to gain advantage and take hold of the gut.
In 2000, Boston-based researchers constructed an E.coli with a genetic switch that toggled between turning genes on and off two. Later, they built some safety checks into their bacteria. “To prevent unintentional or deleterious consequences, in 2009, we built a safety switch in the engineered bacteria’s genetic circuit that gets triggered after it gets exposed to a pathogen," says James Collins, a professor of biological engineering at MIT and faculty member at Harvard University’s Wyss Institute. “After getting rid of the pathogen, the engineered bacteria is designed to switch off and leave the patient's body.”
Overuse and misuse of antibiotics causes resistant strains to evolve
Adobe Stock
Seek and destroy
As the field of synthetic biology developed, scientists began using engineered bacteria to tackle superbugs. They first focused on Vibrio cholerae, which in the 19th and 20th century caused cholera pandemics in India, China, the Middle East, Europe, and Americas. Like many other bacteria, V. cholerae communicate with each other via quorum sensing, a process in which the microorganisms release different signaling molecules, to convey messages to its brethren. Highly intelligent by bacterial standards, some multidrug resistant V. cholerae strains can also “collaborate” with other intestinal bacterial species to gain advantage and take hold of the gut. When untreated, cholera has a mortality rate of 25 to 50 percent and outbreaks frequently occur in developing countries, especially during floods and droughts.
Sometimes, however, V. cholerae makes mistakes. In 2008, researchers at Cornell University observed that when quorum sensing V. cholerae accidentally released high concentrations of a signaling molecule called CAI-1, it had a counterproductive effect—the pathogen couldn’t colonize the gut.
So the group, led by John March, professor of biological and environmental engineering, developed a novel strategy to combat V. cholerae. They genetically engineered E.coli to eavesdrop on V. cholerae communication networks and equipped it with the ability to release the CAI-1 molecules. That interfered with V. cholerae progress. Two years later, the Cornell team showed that V. cholerae-infected mice treated with engineered E.coli had a 92 percent survival rate.
These findings inspired researchers to sic the good bacteria present in foods like yogurt and kimchi onto the drug-resistant ones.
Three years later in 2011, Singapore-based scientists engineered E.coli to detect and destroy Pseudomonas aeruginosa, an often drug-resistant pathogen that causes pneumonia, urinary tract infections, and sepsis. Once the genetically engineered E.coli found its target through its quorum sensing molecules, it then released a peptide, that could eradicate 99 percent of P. aeruginosa cells in a test-tube experiment. The team outlined their work in a Molecular Systems Biology study.
“At the time, we knew that we were entering new, uncharted territory,” says lead author Matthew Chang, an associate professor and synthetic biologist at the National University of Singapore and lead author of the study. “To date, we are still in the process of trying to understand how long these microbes stay in our bodies and how they might continue to evolve.”
More teams followed the same path. In a 2013 study, MIT researchers also genetically engineered E.coli to detect P. aeruginosa via the pathogen’s quorum-sensing molecules. It then destroyed the pathogen by secreting a lab-made toxin.
Probiotics that fight
A year later in 2014, a Nature study found that the abundance of Ruminococcus obeum, a probiotic bacteria naturally occurring in the human microbiome, interrupts and reduces V.cholerae’s colonization— by detecting the pathogen’s quorum sensing molecules. The natural accumulation of R. obeum in Bangladeshi adults helped them recover from cholera despite living in an area with frequent outbreaks.
The findings from 2008 to 2014 inspired Collins and his team to delve into how good bacteria present in foods like yogurt and kimchi can attack drug-resistant bacteria. In 2018, Collins and his team developed the engineered probiotic strategy. They tweaked a bacteria commonly found in yogurt called Lactococcus lactis to treat cholera.
Engineered bacteria can be trained to target pathogens when they are at their most vulnerable metabolic stage in the human gut. --José Rubén Morones-Ramírez.
More scientists followed with more experiments. So far, researchers have engineered various probiotic organisms to fight pathogenic bacteria like Staphylococcus aureus (leading cause of skin, tissue, bone, joint and blood infections) and Clostridium perfringens (which causes watery diarrhea) in test-tube and animal experiments. In 2020, Russian scientists engineered a probiotic called Pichia pastoris to produce an enzyme called lysostaphin that eradicated S. aureus in vitro. Another 2020 study from China used an engineered probiotic bacteria Lactobacilli casei as a vaccine to prevent C. perfringens infection in rabbits.
In a study last year, Ramírez’s group at the Autonomous University of Nuevo León, engineered E. coli to detect quorum-sensing molecules from Methicillin-resistant Staphylococcus aureus or MRSA, a notorious superbug. The E. coli then releases a bacteriocin that kills MRSA. “An antibiotic is just a molecule that is not intelligent,” says Ramírez. “On the other hand, engineered bacteria can be trained to target pathogens when they are at their most vulnerable metabolic stage in the human gut.”
Collins and Timothy Lu, an associate professor of biological engineering at MIT, found that engineered E. coli can help treat other conditions—such as phenylketonuria, a rare metabolic disorder, that causes the build-up of an amino acid phenylalanine. Their start-up Synlogic aims to commercialize the technology, and has completed a phase 2 clinical trial.
Circumventing the challenges
The bacteria-engineering technique is not without pitfalls. One major challenge is that beneficial gut bacteria produce their own quorum-sensing molecules that can be similar to those that pathogens secrete. If an engineered bacteria’s biosensor is not specific enough, it will be ineffective.
Another concern is whether engineered bacteria might mutate after entering the gut. “As with any technology, there are risks where bad actors could have the capability to engineer a microbe to act quite nastily,” says Collins of MIT. But Collins and Ramírez both insist that the chances of the engineered bacteria mutating on its own are virtually non-existent. “It is extremely unlikely for the engineered bacteria to mutate,” Ramírez says. “Coaxing a living cell to do anything on command is immensely challenging. Usually, the greater risk is that the engineered bacteria entirely lose its functionality.”
However, the biggest challenge is bringing the curative bacteria to consumers. Pharmaceutical companies aren’t interested in antibiotics or their alternatives because it’s less profitable than developing new medicines for non-infectious diseases. Unlike the more chronic conditions like diabetes or cancer that require long-term medications, infectious diseases are usually treated much quicker. Running clinical trials are expensive and antibiotic-alternatives aren’t lucrative enough.
“Unfortunately, new medications for antibiotic resistant infections have been pushed to the bottom of the field,” says Lu of MIT. “It's not because the technology does not work. This is more of a market issue. Because clinical trials cost hundreds of millions of dollars, the only solution is that governments will need to fund them.” Lu stresses that societies must lobby to change how the modern healthcare industry works. “The whole world needs better treatments for antibiotic resistance.”
Important findings are starting to emerge from research on how genes shape the human response to the Covid virus.
From infections with no symptoms to why men are more likely to be hospitalized in the ICU and die of COVID-19, new research shows that your genes play a significant role
Early in the pandemic, genetic research focused on the virus because it was readily available. Plus, the virus contains only 30,000 bases in a dozen functional genes, so it's relatively easy and affordable to sequence. Additionally, the rapid mutation of the virus and its ability to escape antibody control fueled waves of different variants and provided a reason to follow viral genetics.
In comparison, there are many more genes of the human immune system and cellular functions that affect viral replication, with about 3.2 billion base pairs. Human studies require samples from large numbers of people, the analysis of each sample is vastly more complex, and sophisticated computer analysis often is required to make sense of the raw data. All of this takes time and large amounts of money, but important findings are beginning to emerge.
Asymptomatics
About half the people exposed to SARS-CoV-2, the virus that causes the COVID-19 disease, never develop symptoms of this disease, or their symptoms are so mild they often go unnoticed. One piece of understanding the phenomena came when researchers showed that exposure to OC43, a common coronavirus that results in symptoms of a cold, generates immune system T cells that also help protect against SARS-CoV-2.
Jill Hollenbach, an immunologist at the University of California at San Francisco, sought to identify the gene behind that immune protection. Most COVID-19 genetic studies are done with the most seriously ill patients because they are hospitalized and thus available. “But 99 percent of people who get it will never see the inside of a hospital for COVID-19,” she says. “They are home, they are not interacting with the health care system.”
Early in the pandemic, when most labs were shut down, she tapped into the National Bone Marrow Donor Program database. It contains detailed information on donor human leukocyte antigens (HLAs), key genes in the immune system that must match up between donor and recipient for successful transplants of marrow or organs. Each HLA can contain alleles, slight molecular differences in the DNA of the HLA, which can affect its function. Potential HLA combinations can number in the tens of thousands across the world, says Hollenbach, but each person has a smaller number of those possible variants.
She teamed up with the COVID-19 Citizen Science Study a smartphone-based study to track COVID-19 symptoms and outcomes, to ask persons in the bone marrow donor registry about COVID-19. The study enlisted more than 30,000 volunteers. Those volunteers already had their HLAs annotated by the registry, and 1,428 tested positive for the virus.
Analyzing five key HLAs, she found an allele in the gene HLA-B*15:01 that was significantly overrepresented in people who didn’t have any symptoms. The effect was even stronger if a person had inherited the allele from both parents; these persons were “more than eight times more likely to remain asymptomatic than persons who did not carry the genetic variant,” she says. Altogether this HLA was present in about 10 percent of the general European population but double that percentage in the asymptomatic group. Hollenbach and her colleagues were able confirm this in other different groups of patients.
What made the allele so potent against SARS-CoV-2? Part of the answer came from x-ray crystallography. A key element was the molecular shape of parts of the cold virus OC43 and SARS-CoV-2. They were virtually identical, and the allele could bind very tightly to them, present their molecular antigens to T cells, and generate an extremely potent T cell response to the viruses. And “for whatever reasons that generated a lot of memory T cells that are going to stick around for a long time,” says Hollenbach. “This T cell response is very early in infection and ramps up very quickly, even before the antibody response.”
Understanding the genetics of the immune response to SARS-CoV-2 is important because it provides clues into the conditions of T cells and antigens that support a response without any symptoms, she says. “It gives us an opportunity to think about whether this might be a vaccine design strategy.”
Dead men
A researcher at the Leibniz Institute of Virology in Hamburg Germany, Guelsah Gabriel, was drawn to a question at the other end of the COVID-19 spectrum: why men more likely to be hospitalized and die from the infection. It wasn't that men were any more likely to be exposed to the virus but more likely, how their immune system reacted to it
Several studies had noted that testosterone levels were significantly lower in men hospitalized with COVID-19. And, in general, the lower the testosterone, the worse the prognosis. A year after recovery, about 30 percent of men still had lower than normal levels of testosterone, a condition known as hypogonadism. Most of the men also had elevated levels of estradiol, a female hormone (https://pubmed.ncbi.nlm.nih.gov/34402750/).
Every cell has a sex, expressing receptors for male and female hormones on their surface. Hormones docking with these receptors affect the cells' internal function and the signals they send to other cells. The number and role of these receptors varies from tissue to tissue.
Gabriel began her search by examining whole exome sequences, the protein-coding part of the genome, for key enzymes involved in the metabolism of sex hormones. The research team quickly zeroed in on CYP19A1, an enzyme that converts testosterone to estradiol. The gene that produces this enzyme has a number of different alleles, the molecular variants that affect the enzyme's rate of metabolizing the sex hormones. One genetic variant, CYP19A1 (Thr201Met), is typically found in 6.2 percent of all people, both men and women, but remarkably, they found it in 68.7 percent of men who were hospitalized with COVID-19.
Lung surprise
Lungs are the tissue most affected in COVID-19 disease. Gabriel wondered if the virus might be affecting expression of their target gene in the lung so that it produces more of the enzyme that converts testosterone to estradiol. Studying cells in a petri dish, they saw no change in gene expression when they infected cells of lung tissue with influenza and the original SARS-CoV viruses that caused the SARS outbreak in 2002. But exposure to SARS-CoV-2, the virus responsible for COVID-19, increased gene expression up to 40-fold, Gabriel says.
Did the same thing happen in humans? Autopsy examination of patients in three different cites found that “CYP19A1 was abundantly expressed in the lungs of COVID-19 males but not those who died of other respiratory infections,” says Gabriel. This increased enzyme production led likely to higher levels of estradiol in the lungs of men, which “is highly inflammatory, damages the tissue, and can result in fibrosis or scarring that inhibits lung function and repair long after the virus itself has disappeared.” Somehow the virus had acquired the capacity to upregulate expression of CYP19A1.
Only two COVID-19 positive females showed increased expression of this gene. The menopause status of these women, or whether they were on hormone replacement therapy was not known. That could be important because female hormones have a protective effect for cardiovascular disease, which women often lose after going through menopause, especially if they don’t start hormone replacement therapy. That sex-specific protection might also extend to COVID-19 and merits further study.
The team was able to confirm their findings in golden hamsters, the animal model of choice for studying COVID-19. Testosterone levels in male animals dropped 5-fold three days after infection and began to recover as viral levels declined. CYP19A1 transcription increased up to 15-fold in the lungs of the male but not the females. The study authors wrote, “Virus replication in the male lungs was negatively associated with testosterone levels.”
The medical community studying COVID-19 has slowly come to recognize the importance of adipose tissue, or fat cells. They are known to express abundant levels of CYP19A1 and play a significant role as metabolic tissue in COVID-19. Gabriel adds, “One of the key findings of our study is that upon SARS-CoV-2 infection, the lung suddenly turns into a metabolic organ by highly expressing” CYP19A1.
She also found evidence that SARS-CoV-2 can infect the gonads of hamsters, thereby likely depressing circulating levels of sex hormones. The researchers did not have autopsy samples to confirm this in humans, but others have shown that the virus can replicate in those tissues.
A possible treatment
Back in the lab, substituting low and high doses of testosterone in SARS-COV-2 infected male hamsters had opposite effects depending on testosterone dosage used. Gabriel says that hormone levels can vary so much, depending on health status and age and even may change throughout the day, that “it probably is much better to inhibit the enzyme” produced by CYP19A1 than try to balance the hormones.
Results were better with letrozole, a drug approved to treat hypogonadism in males, which reduces estradiol levels. The drug also showed benefit in male hamsters in terms of less severe disease and faster recovery. She says more details need to be worked out in using letrozole to treat COVID-19, but they are talking with hospitals about clinical trials of the drug.
Gabriel has proposed a four hit explanation of how COVID-19 can be so deadly for men: the metabolic quartet. First is the genetic risk factor of CYP19A1 (Thr201Met), then comes SARS-CoV-2 infection that induces even greater expression of this gene and the deleterious increase of estradiol in the lung. Age-related hypogonadism and the heightened inflammation of obesity, known to affect CYP19A1 activity, are contributing factors in this deadly perfect storm of events.
Studying host genetics, says Gabriel, can reveal new mechanisms that yield promising avenues for further study. It’s also uniting different fields of science into a new, collaborative approach they’re calling “infection endocrinology,” she says.
New device finds breast cancer like earthquake detection
Jessica Fitzjohn, a postdoctoral fellow at the University of Canterbury, demonstrates the novel breast cancer screening device.
Mammograms are necessary breast cancer checks for women as they reach the recommended screening age between 40 and 50 years. Yet, many find the procedure uncomfortable. “I have large breasts, and to be able to image the full breast, the radiographer had to manipulate my breast within the machine, which took time and was quite uncomfortable,” recalls Angela, who preferred not to disclose her last name.
Breast cancer is the most widespread cancer in the world, affecting 2.3 million women in 2020. Screening exams such as mammograms can help find breast cancer early, leading to timely diagnosis and treatment. If this type of cancer is detected before the disease has spread, the 5-year survival rate is 99 percent. But some women forgo mammograms due to concerns about radiation or painful compression of breasts. Other issues, such as low income and a lack of access to healthcare, can also serve as barriers, especially for underserved populations.
Researchers at the University of Canterbury and startup Tiro Medical in Christchurch, New Zealand are hoping their new device—which doesn’t involve any radiation or compression of the breasts—could increase the accuracy of breast cancer screening, broaden access and encourage more women to get checked. They’re digging into clues from the way buildings move in an earthquake to help detect more cases of this disease.
Earthquake engineering inspires new breast cancer screening tech
What’s underneath a surface affects how it vibrates. Earthquake engineers look at the vibrations of swaying buildings to identify the underlying soil and tissue properties. “As the vibration wave travels, it reflects the stiffness of the material between that wave and the surface,” says Geoff Chase, professor of engineering at the University of Canterbury in Christchurch, New Zealand.
Chase is applying this same concept to breasts. Analyzing the surface motion of the breast as it vibrates could reveal the stiffness of the tissues underneath. Regions of high stiffness could point to cancer, given that cancerous breast tissue can be up to 20 times stiffer than normal tissue. “If in essence every woman’s breast is soft soil, then if you have some granite rocks in there, we’re going to see that on the surface,” explains Chase.
The earthquake-inspired device exceeds the 87 percent sensitivity of a 3D mammogram.
That notion underpins a new breast screening device, the brainchild of Chase. Women lie face down, with their breast being screened inside a circular hole and the nipple resting on a small disc called an actuator. The actuator moves up and down, between one and two millimeters, so there’s a small vibration, “almost like having your phone vibrate on your nipple,” says Jessica Fitzjohn, a postdoctoral fellow at the University of Canterbury who collaborated on the device design with Chase.
Cameras surrounding the device take photos of the breast surface motion as it vibrates. The photos are fed into image processing algorithms that convert them into data points. Then, diagnostic algorithms analyze those data points to find any differences in the breast tissue. “We’re looking for that stiffness contrast which could indicate a tumor,” Fitzjohn says.
A nascent yet promising technology
The device has been tested in a clinical trial of 14 women: one with healthy breasts and 13 with a tumor in one breast. The cohort was small but diverse, varying in age, breast volume and tumor size.
Results from the trial yielded a sensitivity rate, or the likelihood of correctly detecting breast cancer, of 85 percent. Meanwhile, the device’s specificity rate, or the probability of diagnosing healthy breasts, was 77 percent. By combining and optimizing certain diagnostic algorithms, the device reached between 92 and 100 percent sensitivity and between 80 and 86 percent specificity, which is comparable to the latest 3D mammogram technology. Called tomosynthesis, these 3D mammograms take a number of sharper, clearer and more detailed 3D images compared to the single 2D image of a conventional mammogram, and have a specificity score of 92 percent. Although the earthquake-inspired device’s specificity is lower, it exceeds the 87 percent sensitivity of a 3D mammogram.
The team hopes that cameras with better resolution can help improve the numbers. And with a limited amount of data in the first trial, the researchers are looking into funding for another clinical trial to validate their results on a larger cohort size.
Additionally, during the trial, the device correctly identified one woman’s breast as healthy, while her prior mammogram gave a false positive. The device correctly identified it as being healthy tissue. It was also able to capture the tiniest tumor at 7 millimeters—around a third of an inch or half as long as an aspirin tablet.
Diagnostic findings from the device are immediate.

When using the earthquake-inspired device, women lie face down, with their breast being screened inside circular holes.
University of Canterbury.
But more testing is needed to “prove the device’s ability to pick up small breast cancers less than 10 to 15 millimeters in size, as we know that finding cancers when they are small is the best way of improving outcomes,” says Richard Annand, a radiologist at Pacific Radiology in New Zealand. He explains that mammography already detects most precancerous lesions, so if the device will only be able to find large masses or lumps it won’t be particularly useful. While not directly involved in administering the clinical trial for the device, Annand was a director at the time for Canterbury Breastcare, where the trial occurred.
Meanwhile, Monique Gary, a breast surgical oncologist and medical director of the Grand View Health Cancer program in Pennsylvania, U.S., is excited to see new technologies advancing breast cancer screening and early detection. But she notes that the device may be challenging for “patients who are unable to lay prone, such as pregnant women as well as those who are differently abled, and this machine might exclude them.” She adds that it would also be interesting to explore how breast implants would impact the device’s vibrational frequency.
Diagnostic findings from the device are immediate, with the results available “before you put your clothes back on,” Chase says. The absence of any radiation is another benefit, though Annand considers it a minor edge “as we know the radiation dose used in mammography is minimal, and the advantages of having a mammogram far outweigh the potential risk of radiation.”
The researchers also conducted a separate ergonomic trial with 40 women to assess the device’s comfort, safety and ease of use. Angela was part of that trial and described the experience as “easy, quick, painless and required no manual intervention from an operator.” And if a person is uncomfortable being topless or having their breasts touched by someone else, “this type of device would make them more comfortable and less exposed,” she says.
While mammograms remain “the ‘gold standard’ in breast imaging, particularly screening, physicians need an option that can be used in combination with mammography.
Fitzjohn acknowledges that “at the moment, it’s quite a crude prototype—it’s just a block that you lie on.” The team prioritized function over form initially, but they’re now planning a few design improvements, including more cushioning for the breasts and the surface where the women lie on.
While mammograms remains “the ‘gold standard’ in breast imaging, particularly screening, physicians need an option that is good at excluding breast cancer when used in combination with mammography, has good availability, is easy to use and is affordable. There is the possibility that the device could fill this role,” Annand says.
Indeed, the researchers envision their new breast screening device as complementary to mammograms—a prescreening tool that could make breast cancer checks widely available. As the device is portable and doesn’t require specialized knowledge to operate, it can be used in clinics, pop-up screening facilities and rural communities. “If it was easily accessible, particularly as part of a checkup with a [general practitioner] or done in a practice the patient is familiar with, it may encourage more women to access this service,” Angela says. For those who find regular mammograms uncomfortable or can’t afford them, the earthquake-inspired device may be an option—and an even better one.
Broadening access could prompt more women to go for screenings, particularly younger women at higher risk of getting breast cancer because of a family history of the disease or specific gene mutations. “If we can provide an option for them then we can catch those cancers earlier,” Fitzjohn syas. “By taking screening to people, we’re increasing patient-centric care.”
With the team aiming to lower the device’s cost to somewhere between five and eight times less than mammography equipment, it would also be valuable for low-to-middle-income nations that are challenged to afford the infrastructure for mammograms or may not have enough skilled radiologists.
For Fitzjohn, the ultimate goal is to “increase equity in breast screening and catch cancer early so we have better outcomes for women who are diagnosed with breast cancer.”