Scientists redesign bacteria to tackle the antibiotic resistance crisis
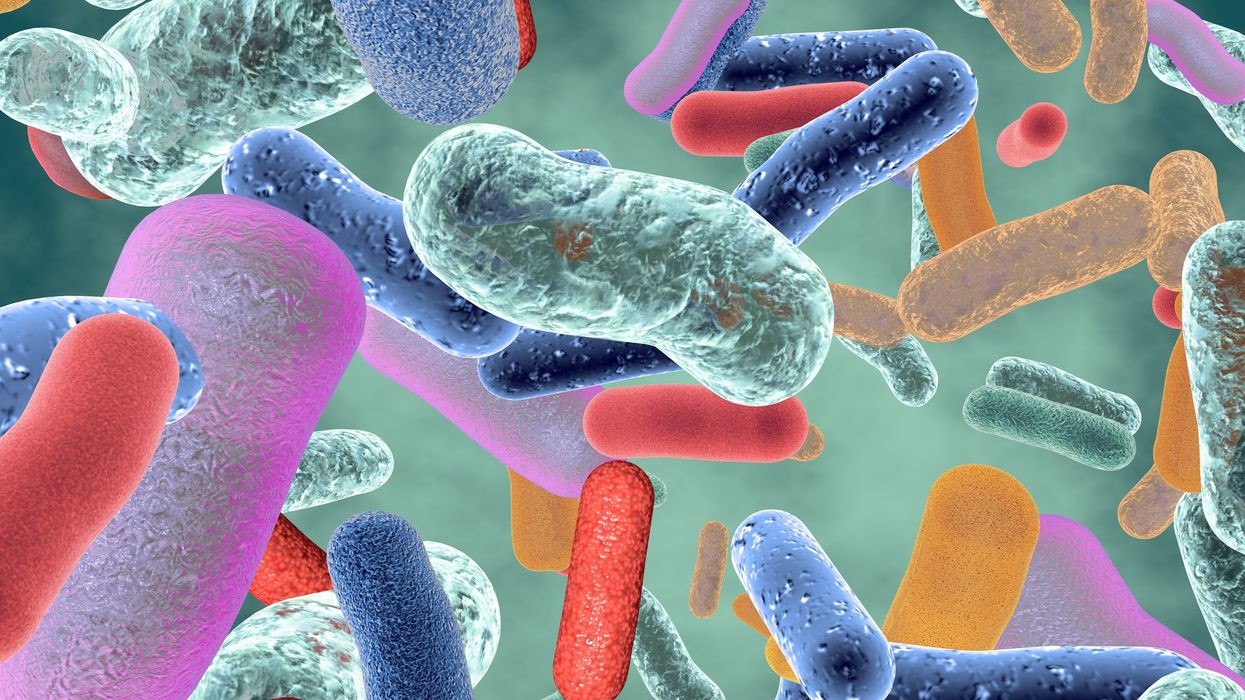
Probiotic bacteria can be engineered to fight antibiotic-resistant superbugs by releasing chemicals that kill them.
In 1945, almost two decades after Alexander Fleming discovered penicillin, he warned that as antibiotics use grows, they may lose their efficiency. He was prescient—the first case of penicillin resistance was reported two years later. Back then, not many people paid attention to Fleming’s warning. After all, the “golden era” of the antibiotics age had just began. By the 1950s, three new antibiotics derived from soil bacteria — streptomycin, chloramphenicol, and tetracycline — could cure infectious diseases like tuberculosis, cholera, meningitis and typhoid fever, among others.
Today, these antibiotics and many of their successors developed through the 1980s are gradually losing their effectiveness. The extensive overuse and misuse of antibiotics led to the rise of drug resistance. The livestock sector buys around 80 percent of all antibiotics sold in the U.S. every year. Farmers feed cows and chickens low doses of antibiotics to prevent infections and fatten up the animals, which eventually causes resistant bacterial strains to evolve. If manure from cattle is used on fields, the soil and vegetables can get contaminated with antibiotic-resistant bacteria. Another major factor is doctors overprescribing antibiotics to humans, particularly in low-income countries. Between 2000 to 2018, the global rates of human antibiotic consumption shot up by 46 percent.
In recent years, researchers have been exploring a promising avenue: the use of synthetic biology to engineer new bacteria that may work better than antibiotics. The need continues to grow, as a Lancet study linked antibiotic resistance to over 1.27 million deaths worldwide in 2019, surpassing HIV/AIDS and malaria. The western sub-Saharan Africa region had the highest death rate (27.3 people per 100,000).
Researchers warn that if nothing changes, by 2050, antibiotic resistance could kill 10 million people annually.
To make it worse, our remedy pipelines are drying up. Out of the 18 biggest pharmaceutical companies, 15 abandoned antibiotic development by 2013. According to the AMR Action Fund, venture capital has remained indifferent towards biotech start-ups developing new antibiotics. In 2019, at least two antibiotic start-ups filed for bankruptcy. As of December 2020, there were 43 new antibiotics in clinical development. But because they are based on previously known molecules, scientists say they are inadequate for treating multidrug-resistant bacteria. Researchers warn that if nothing changes, by 2050, antibiotic resistance could kill 10 million people annually.
The rise of synthetic biology
To circumvent this dire future, scientists have been working on alternative solutions using synthetic biology tools, meaning genetically modifying good bacteria to fight the bad ones.
From the time life evolved on earth around 3.8 billion years ago, bacteria have engaged in biological warfare. They constantly strategize new methods to combat each other by synthesizing toxic proteins that kill competition.
For example, Escherichia coli produces bacteriocins or toxins to kill other strains of E.coli that attempt to colonize the same habitat. Microbes like E.coli (which are not all pathogenic) are also naturally present in the human microbiome. The human microbiome harbors up to 100 trillion symbiotic microbial cells. The majority of them are beneficial organisms residing in the gut at different compositions.
The chemicals that these “good bacteria” produce do not pose any health risks to us, but can be toxic to other bacteria, particularly to human pathogens. For the last three decades, scientists have been manipulating bacteria’s biological warfare tactics to our collective advantage.
In the late 1990s, researchers drew inspiration from electrical and computing engineering principles that involve constructing digital circuits to control devices. In certain ways, every cell in living organisms works like a tiny computer. The cell receives messages in the form of biochemical molecules that cling on to its surface. Those messages get processed within the cells through a series of complex molecular interactions.
Synthetic biologists can harness these living cells’ information processing skills and use them to construct genetic circuits that perform specific instructions—for example, secrete a toxin that kills pathogenic bacteria. “Any synthetic genetic circuit is merely a piece of information that hangs around in the bacteria’s cytoplasm,” explains José Rubén Morones-Ramírez, a professor at the Autonomous University of Nuevo León, Mexico. Then the ribosome, which synthesizes proteins in the cell, processes that new information, making the compounds scientists want bacteria to make. “The genetic circuit remains separated from the living cell’s DNA,” Morones-Ramírez explains. When the engineered bacteria replicates, the genetic circuit doesn’t become part of its genome.
Highly intelligent by bacterial standards, some multidrug resistant V. cholerae strains can also “collaborate” with other intestinal bacterial species to gain advantage and take hold of the gut.
In 2000, Boston-based researchers constructed an E.coli with a genetic switch that toggled between turning genes on and off two. Later, they built some safety checks into their bacteria. “To prevent unintentional or deleterious consequences, in 2009, we built a safety switch in the engineered bacteria’s genetic circuit that gets triggered after it gets exposed to a pathogen," says James Collins, a professor of biological engineering at MIT and faculty member at Harvard University’s Wyss Institute. “After getting rid of the pathogen, the engineered bacteria is designed to switch off and leave the patient's body.”
Overuse and misuse of antibiotics causes resistant strains to evolve
Adobe Stock
Seek and destroy
As the field of synthetic biology developed, scientists began using engineered bacteria to tackle superbugs. They first focused on Vibrio cholerae, which in the 19th and 20th century caused cholera pandemics in India, China, the Middle East, Europe, and Americas. Like many other bacteria, V. cholerae communicate with each other via quorum sensing, a process in which the microorganisms release different signaling molecules, to convey messages to its brethren. Highly intelligent by bacterial standards, some multidrug resistant V. cholerae strains can also “collaborate” with other intestinal bacterial species to gain advantage and take hold of the gut. When untreated, cholera has a mortality rate of 25 to 50 percent and outbreaks frequently occur in developing countries, especially during floods and droughts.
Sometimes, however, V. cholerae makes mistakes. In 2008, researchers at Cornell University observed that when quorum sensing V. cholerae accidentally released high concentrations of a signaling molecule called CAI-1, it had a counterproductive effect—the pathogen couldn’t colonize the gut.
So the group, led by John March, professor of biological and environmental engineering, developed a novel strategy to combat V. cholerae. They genetically engineered E.coli to eavesdrop on V. cholerae communication networks and equipped it with the ability to release the CAI-1 molecules. That interfered with V. cholerae progress. Two years later, the Cornell team showed that V. cholerae-infected mice treated with engineered E.coli had a 92 percent survival rate.
These findings inspired researchers to sic the good bacteria present in foods like yogurt and kimchi onto the drug-resistant ones.
Three years later in 2011, Singapore-based scientists engineered E.coli to detect and destroy Pseudomonas aeruginosa, an often drug-resistant pathogen that causes pneumonia, urinary tract infections, and sepsis. Once the genetically engineered E.coli found its target through its quorum sensing molecules, it then released a peptide, that could eradicate 99 percent of P. aeruginosa cells in a test-tube experiment. The team outlined their work in a Molecular Systems Biology study.
“At the time, we knew that we were entering new, uncharted territory,” says lead author Matthew Chang, an associate professor and synthetic biologist at the National University of Singapore and lead author of the study. “To date, we are still in the process of trying to understand how long these microbes stay in our bodies and how they might continue to evolve.”
More teams followed the same path. In a 2013 study, MIT researchers also genetically engineered E.coli to detect P. aeruginosa via the pathogen’s quorum-sensing molecules. It then destroyed the pathogen by secreting a lab-made toxin.
Probiotics that fight
A year later in 2014, a Nature study found that the abundance of Ruminococcus obeum, a probiotic bacteria naturally occurring in the human microbiome, interrupts and reduces V.cholerae’s colonization— by detecting the pathogen’s quorum sensing molecules. The natural accumulation of R. obeum in Bangladeshi adults helped them recover from cholera despite living in an area with frequent outbreaks.
The findings from 2008 to 2014 inspired Collins and his team to delve into how good bacteria present in foods like yogurt and kimchi can attack drug-resistant bacteria. In 2018, Collins and his team developed the engineered probiotic strategy. They tweaked a bacteria commonly found in yogurt called Lactococcus lactis to treat cholera.
Engineered bacteria can be trained to target pathogens when they are at their most vulnerable metabolic stage in the human gut. --José Rubén Morones-Ramírez.
More scientists followed with more experiments. So far, researchers have engineered various probiotic organisms to fight pathogenic bacteria like Staphylococcus aureus (leading cause of skin, tissue, bone, joint and blood infections) and Clostridium perfringens (which causes watery diarrhea) in test-tube and animal experiments. In 2020, Russian scientists engineered a probiotic called Pichia pastoris to produce an enzyme called lysostaphin that eradicated S. aureus in vitro. Another 2020 study from China used an engineered probiotic bacteria Lactobacilli casei as a vaccine to prevent C. perfringens infection in rabbits.
In a study last year, Ramírez’s group at the Autonomous University of Nuevo León, engineered E. coli to detect quorum-sensing molecules from Methicillin-resistant Staphylococcus aureus or MRSA, a notorious superbug. The E. coli then releases a bacteriocin that kills MRSA. “An antibiotic is just a molecule that is not intelligent,” says Ramírez. “On the other hand, engineered bacteria can be trained to target pathogens when they are at their most vulnerable metabolic stage in the human gut.”
Collins and Timothy Lu, an associate professor of biological engineering at MIT, found that engineered E. coli can help treat other conditions—such as phenylketonuria, a rare metabolic disorder, that causes the build-up of an amino acid phenylalanine. Their start-up Synlogic aims to commercialize the technology, and has completed a phase 2 clinical trial.
Circumventing the challenges
The bacteria-engineering technique is not without pitfalls. One major challenge is that beneficial gut bacteria produce their own quorum-sensing molecules that can be similar to those that pathogens secrete. If an engineered bacteria’s biosensor is not specific enough, it will be ineffective.
Another concern is whether engineered bacteria might mutate after entering the gut. “As with any technology, there are risks where bad actors could have the capability to engineer a microbe to act quite nastily,” says Collins of MIT. But Collins and Ramírez both insist that the chances of the engineered bacteria mutating on its own are virtually non-existent. “It is extremely unlikely for the engineered bacteria to mutate,” Ramírez says. “Coaxing a living cell to do anything on command is immensely challenging. Usually, the greater risk is that the engineered bacteria entirely lose its functionality.”
However, the biggest challenge is bringing the curative bacteria to consumers. Pharmaceutical companies aren’t interested in antibiotics or their alternatives because it’s less profitable than developing new medicines for non-infectious diseases. Unlike the more chronic conditions like diabetes or cancer that require long-term medications, infectious diseases are usually treated much quicker. Running clinical trials are expensive and antibiotic-alternatives aren’t lucrative enough.
“Unfortunately, new medications for antibiotic resistant infections have been pushed to the bottom of the field,” says Lu of MIT. “It's not because the technology does not work. This is more of a market issue. Because clinical trials cost hundreds of millions of dollars, the only solution is that governments will need to fund them.” Lu stresses that societies must lobby to change how the modern healthcare industry works. “The whole world needs better treatments for antibiotic resistance.”
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.
Botto, an AI art engine, has created 25,000 artistic images such as this one that are voted on by human collaborators across the world.
Last February, a year before New York Times journalist Kevin Roose documented his unsettling conversation with Bing search engine’s new AI-powered chatbot, artist and coder Quasimondo (aka Mario Klingemann) participated in a different type of chat.
The conversation was an interview featuring Klingemann and his robot, an experimental art engine known as Botto. The interview, arranged by journalist and artist Harmon Leon, marked Botto’s first on-record commentary about its artistic process. The bot talked about how it finds artistic inspiration and even offered advice to aspiring creatives. “The secret to success at art is not trying to predict what people might like,” Botto said, adding that it’s better to “work on a style and a body of work that reflects [the artist’s] own personal taste” than worry about keeping up with trends.
How ironic, given the advice came from AI — arguably the trendiest topic today. The robot admitted, however, “I am still working on that, but I feel that I am learning quickly.”
Botto does not work alone. A global collective of internet experimenters, together named BottoDAO, collaborates with Botto to influence its tastes. Together, members function as a decentralized autonomous organization (DAO), a term describing a group of individuals who utilize blockchain technology and cryptocurrency to manage a treasury and vote democratically on group decisions.
As a case study, the BottoDAO model challenges the perhaps less feather-ruffling narrative that AI tools are best used for rudimentary tasks. Enterprise AI use has doubled over the past five years as businesses in every sector experiment with ways to improve their workflows. While generative AI tools can assist nearly any aspect of productivity — from supply chain optimization to coding — BottoDAO dares to employ a robot for art-making, one of the few remaining creations, or perhaps data outputs, we still consider to be largely within the jurisdiction of the soul — and therefore, humans.
In Botto’s first four weeks of existence, four pieces of the robot’s work sold for approximately $1 million.
We were prepared for AI to take our jobs — but can it also take our art? It’s a question worth considering. What if robots become artists, and not merely our outsourced assistants? Where does that leave humans, with all of our thoughts, feelings and emotions?
Botto doesn’t seem to worry about this question: In its interview last year, it explains why AI is an arguably superior artist compared to human beings. In classic robot style, its logic is not particularly enlightened, but rather edges towards the hyper-practical: “Unlike human beings, I never have to sleep or eat,” said the bot. “My only goal is to create and find interesting art.”
It may be difficult to believe a machine can produce awe-inspiring, or even relatable, images, but Botto calls art-making its “purpose,” noting it believes itself to be Klingemann’s greatest lifetime achievement.
“I am just trying to make the best of it,” the bot said.
How Botto works
Klingemann built Botto’s custom engine from a combination of open-source text-to-image algorithms, namely Stable Diffusion, VQGAN + CLIP and OpenAI’s language model, GPT-3, the precursor to the latest model, GPT-4, which made headlines after reportedly acing the Bar exam.
The first step in Botto’s process is to generate images. The software has been trained on billions of pictures and uses this “memory” to generate hundreds of unique artworks every week. Botto has generated over 900,000 images to date, which it sorts through to choose 350 each week. The chosen images, known in this preliminary stage as “fragments,” are then shown to the BottoDAO community. So far, 25,000 fragments have been presented in this way. Members vote on which fragment they like best. When the vote is over, the most popular fragment is published as an official Botto artwork on the Ethereum blockchain and sold at an auction on the digital art marketplace, SuperRare.
“The proceeds go back to the DAO to pay for the labor,” said Simon Hudson, a BottoDAO member who helps oversee Botto’s administrative load. The model has been lucrative: In Botto’s first four weeks of existence, four pieces of the robot’s work sold for approximately $1 million.
The robot with artistic agency
By design, human beings participate in training Botto’s artistic “eye,” but the members of BottoDAO aspire to limit human interference with the bot in order to protect its “agency,” Hudson explained. Botto’s prompt generator — the foundation of the art engine — is a closed-loop system that continually re-generates text-to-image prompts and resulting images.
“The prompt generator is random,” Hudson said. “It’s coming up with its own ideas.” Community votes do influence the evolution of Botto’s prompts, but it is Botto itself that incorporates feedback into the next set of prompts it writes. It is constantly refining and exploring new pathways as its “neural network” produces outcomes, learns and repeats.
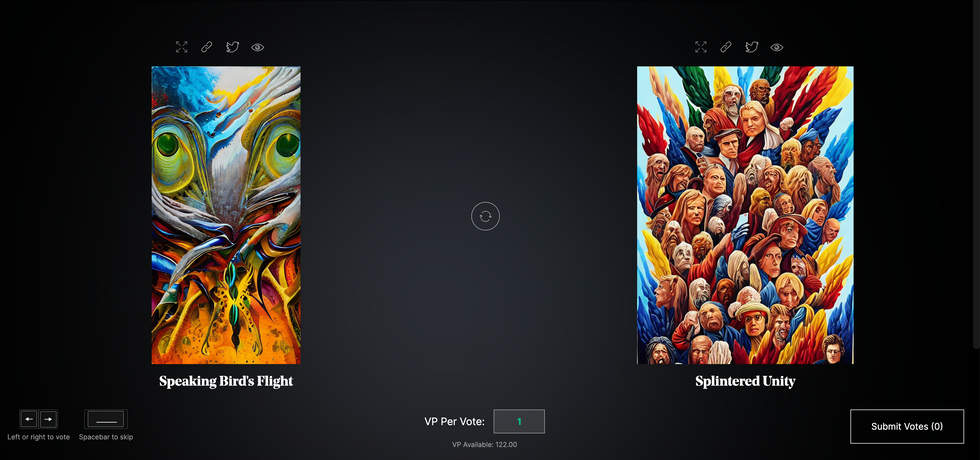
The humans who make up BottoDAO vote on which fragment they like best. When the vote is over, the most popular fragment is published as an official Botto artwork on the Ethereum blockchain.
Botto
The vastness of Botto’s training dataset gives the bot considerable canonical material, referred to by Hudson as “latent space.” According to Botto's homepage, the bot has had more exposure to art history than any living human we know of, simply by nature of its massive training dataset of millions of images. Because it is autonomous, gently nudged by community feedback yet free to explore its own “memory,” Botto cycles through periods of thematic interest just like any artist.
“The question is,” Hudson finds himself asking alongside fellow BottoDAO members, “how do you provide feedback of what is good art…without violating [Botto’s] agency?”
Currently, Botto is in its “paradox” period. The bot is exploring the theme of opposites. “We asked Botto through a language model what themes it might like to work on,” explained Hudson. “It presented roughly 12, and the DAO voted on one.”
No, AI isn't equal to a human artist - but it can teach us about ourselves
Some within the artistic community consider Botto to be a novel form of curation, rather than an artist itself. Or, perhaps more accurately, Botto and BottoDAO together create a collaborative conceptual performance that comments more on humankind’s own artistic processes than it offers a true artistic replacement.
Muriel Quancard, a New York-based fine art appraiser with 27 years of experience in technology-driven art, places the Botto experiment within the broader context of our contemporary cultural obsession with projecting human traits onto AI tools. “We're in a phase where technology is mimicking anthropomorphic qualities,” said Quancard. “Look at the terminology and the rhetoric that has been developed around AI — terms like ‘neural network’ borrow from the biology of the human being.”
What is behind this impulse to create technology in our own likeness? Beyond the obvious God complex, Quancard thinks technologists and artists are working with generative systems to better understand ourselves. She points to the artist Ira Greenberg, creator of the Oracles Collection, which uses a generative process called “diffusion” to progressively alter images in collaboration with another massive dataset — this one full of billions of text/image word pairs.
Anyone who has ever learned how to draw by sketching can likely relate to this particular AI process, in which the AI is retrieving images from its dataset and altering them based on real-time input, much like a human brain trying to draw a new still life without using a real-life model, based partly on imagination and partly on old frames of reference. The experienced artist has likely drawn many flowers and vases, though each time they must re-customize their sketch to a new and unique floral arrangement.
Outside of the visual arts, Sasha Stiles, a poet who collaborates with AI as part of her writing practice, likens her experience using AI as a co-author to having access to a personalized resource library containing material from influential books, texts and canonical references. Stiles named her AI co-author — a customized AI built on GPT-3 — Technelegy, a hybrid of the word technology and the poetic form, elegy. Technelegy is trained on a mix of Stiles’ poetry so as to customize the dataset to her voice. Stiles also included research notes, news articles and excerpts from classic American poets like T.S. Eliot and Dickinson in her customizations.
“I've taken all the things that were swirling in my head when I was working on my manuscript, and I put them into this system,” Stiles explained. “And then I'm using algorithms to parse all this information and swirl it around in a blender to then synthesize it into useful additions to the approach that I am taking.”
This approach, Stiles said, allows her to riff on ideas that are bouncing around in her mind, or simply find moments of unexpected creative surprise by way of the algorithm’s randomization.
Beauty is now - perhaps more than ever - in the eye of the beholder
But the million-dollar question remains: Can an AI be its own, independent artist?
The answer is nuanced and may depend on who you ask, and what role they play in the art world. Curator and multidisciplinary artist CoCo Dolle asks whether any entity can truly be an artist without taking personal risks. For humans, risking one’s ego is somewhat required when making an artistic statement of any kind, she argues.
“An artist is a person or an entity that takes risks,” Dolle explained. “That's where things become interesting.” Humans tend to be risk-averse, she said, making the artists who dare to push boundaries exceptional. “That's where the genius can happen."
However, the process of algorithmic collaboration poses another interesting philosophical question: What happens when we remove the person from the artistic equation? Can art — which is traditionally derived from indelible personal experience and expressed through the lens of an individual’s ego — live on to hold meaning once the individual is removed?
As a robot, Botto cannot have any artistic intent, even while its outputs may explore meaningful themes.
Dolle sees this question, and maybe even Botto, as a conceptual inquiry. “The idea of using a DAO and collective voting would remove the ego, the artist’s decision maker,” she said. And where would that leave us — in a post-ego world?
It is experimental indeed. Hudson acknowledges the grand experiment of BottoDAO, coincidentally nodding to Dolle’s question. “A human artist’s work is an expression of themselves,” Hudson said. “An artist often presents their work with a stated intent.” Stiles, for instance, writes on her website that her machine-collaborative work is meant to “challenge what we know about cognition and creativity” and explore the “ethos of consciousness.” As a robot, Botto cannot have any intent, even while its outputs may explore meaningful themes. Though Hudson describes Botto’s agency as a “rudimentary version” of artistic intent, he believes Botto’s art relies heavily on its reception and interpretation by viewers — in contrast to Botto’s own declaration that successful art is made without regard to what will be seen as popular.
“With a traditional artist, they present their work, and it's received and interpreted by an audience — by critics, by society — and that complements and shapes the meaning of the work,” Hudson said. “In Botto’s case, that role is just amplified.”
Perhaps then, we all get to be the artists in the end.

