Medical Breakthroughs Set to be Fast-Tracked by Innovative New Health Agency

Pippy Rogers, second from left, with her four siblings, who worry that they are at risk for Alzheimer's and are calling for an acceleration of research.
In 2007, Matthew Might's son, Bertrand, was born with a life-threatening disease that was so rare, doctors couldn't diagnose it. Might, a computer scientist and biologist, eventually realized, "Oh my gosh, he's the only patient in the world with this disease right now." To find effective treatments, new methodologies would need to be developed. But there was no process or playbook for doing that.
Might took it upon himself, along with a team of specialists, to try to find a cure. "What Bertrand really taught me was the visceral sense of urgency when there's suffering, and how to act on that," he said.
He calls it "the agency of urgency"—and patients with more common diseases, such as cancer and Alzheimer's, often feel that same need to take matters into their own hands, as they find their hopes for new treatments running up against bureaucratic systems designed to advance in small, steady steps, not leaps and bounds. "We all hope for a cure," said Florence "Pippy" Rogers, a 65-year-old volunteer with Georgia's chapter of the Alzheimer's Association. She lost her mother to the disease and, these days, worries about herself and her four siblings. "We need to keep accelerating research."
We have a fresh example of what can be achieved by fast-tracking discoveries in healthcare: Covid-19 vaccines.
President Biden has pushed for cancer moonshots since the disease took the life of his son, Beau, in 2015. His administration has now requested $6.5 billion to start a new agency in 2022, called the Advanced Research Projects Agency for Health, or ARPA-H, within the National Institutes of Health. It's based on DARPA, the Department of Defense agency known for hatching world-changing technologies such as drones, GPS and ARPANET, which became the internet.
We have a fresh example of what can be achieved by fast-tracking discoveries in healthcare: Covid-19 vaccines. "Operation Warp Speed was using ARPA-like principles," said Might. "It showed that in a moment of crisis, institutions like NIH can think in an ARPA-like way. So now the question is, why don't we do that all the time?"
But applying the DARPA model to health involves several challenging decisions. I asked experts what could be the hardest question facing advocates of ARPA-H: which health problems it should seek to address. "All the wonderful choices lead to the problem of which ones to choose and prioritize," said Sudip Parikh, CEO of the American Association for the Advancement of Science and executive publisher of the Science family of journals. "There is no objectively right answer."
The Agency of Urgency
ARPA-H will borrow at least three critical ingredients from DARPA: goal-oriented project managers, many from industry; aggressive public-private partnerships; and collaboration among fields that don't always interact. The DARPA concept has been applied to other purposes, including energy and homeland security, with promising results. "We're learning that 'ARPA-ism' is a franchisable model," said Might, a former principal investigator on DARPA projects.
The federal government already pours billions of dollars into advancing research on life-threatening diseases, with much of it channeled through the National Institutes of Health. But the purpose of ARPA-H "isn't just the usual suspects that NIH would fund," said David Walt, a Harvard biochemist, an innovator in gene sequencing and former chair of DARPA's Defense Science Research Council. Whereas some NIH-funded studies aim to gradually improve our understanding of diseases, ARPA-H projects will give full focus to real-world applications; they'll use essential findings from NIH research as starting points, drawing from them to rapidly engineer new technologies that could save lives.
And, ultimately, billions in healthcare costs, if ARPA-H lives up to its predecessor's track record; DARPA's breakthroughs have been economic game-changers, while its fail-fast approach—quickly pulling the plug on projects that aren't panning out—helps to avoid sunken costs. ARPA-H could fuel activities similar to the human genome project, which used existing research to map the base pairs that make up DNA, opening new doors for the biotech industry, sparking economic growth and creating hundreds of thousands of new jobs.
Despite a nearly $4 trillion health economy, "we aren't innovating when it comes to technological capabilities for health," said Liz Feld, president of the Suzanne Wright Foundation for pancreatic cancer.
Individual Diseases Ripe for Innovation
Although the need for innovation is clear, which diseases ARPA-H should tackle is less apparent. One important consideration when choosing health priorities could be "how many people suffer from a disease," said Nancy Kass, a professor of bioethics and public health at Johns Hopkins.
That perspective could justify cancer as a top objective. Cancer and heart disease have long been the two major killers in the U.S. Leonidas Platanias, professor of oncology at Northwestern and director of its cancer center, noted that we've already made significant progress on heart disease. "Anti-cholesterol drugs really have a wide impact," he said. "I don't want to compare one disease to another, but I think cancer may be the most challenging. We need even bigger breakthroughs." He wondered whether ARPA-H should be linked to the part of NIH dedicated to cancer, the National Cancer Institute, "to take maximum advantage of what happens" there.
Previous cancer moonshots have laid a foundation for success. And this sort of disease-by-disease approach makes sense in a way. "We know that concentrating on some diseases has led to treatments," said Parikh. "Think of spinal muscular atrophy or cystic fibrosis. Now, imagine if immune therapies were discovered ten years earlier."
But many advocates think ARPA-H should choose projects that don't revolve around any one disease. "It absolutely has to be disease agnostic," said Feld, president of the pancreatic cancer foundation. "We cannot reach ARPA-H's potential if it's subject to the advocacy of individual patient groups who think their disease is worse than the guy's disease next to them. That's not the way the DARPA model works." Platanias agreed that ARPA-H should "pick the highest concepts and developments that have the best chance" of success.
Finding Connections Between Diseases
Kass, the Hopkins bioethicist, believes that ARPA-H should walk a balance, with some projects focusing on specific diseases and others aspiring to solutions with broader applications, spanning multiple diseases. Being impartial, some have noted, might involve looking at the total "life years" saved by a health innovation; the more diseases addressed by a given breakthrough, the more years of healthy living it may confer. The social and economic value should increase as well.
For multiple payoffs, ARPA-H could concentrate on rare diseases, which can yield important insights for many other diseases, said Might. Every case of cancer and Alzheimer's is, in a way, its own rare disease. Cancer is a genetic disease, like his son Bertrand's rare disorder, and mutations vary widely across cancer patients. "It's safe to say that no two people have ever actually had the same cancer," said Might. In theory, solutions for rare diseases could help us understand how to individualize treatments for more common diseases.
Many experts I talked with support another priority for ARPA-H with implications for multiple diseases: therapies that slow down the aging process. "Aging is the greatest risk factor for every major disease that NIH is studying," said Matt Kaeberlein, a bio-gerontologist at the University of Washington. Yet, "half of one percent of the NIH budget goes to researching the biology of aging. An ARPA-H sized budget would push the field forward at a pace that's hard to imagine."
Might agreed. "It could take ARPA-H to get past the weird stigmas around aging-related research. It could have a tremendous impact on the field."
For example, ARPA-H could try to use mRNA technology to express proteins that affect biological aging, said Kaeberlein. It's an engineering project well-suited to the DARPA model. So is harnessing machine learning to identify biomarkers that assess how fast people are aging. Biological aging clocks, if validated, could quickly reveal whether proposed therapies for aging are working or not. "I think there's huge value in that," said Kaeberlein.
By delivering breakthroughs in computation, ARPA-H could improve diagnostics for many different diseases. That could include improving biowearables for continuously monitoring blood pressure—a hypothetical mentioned in the White House's concept paper on ARPA-H—and advanced imaging technologies. "The high cost of medical imaging is a leading reason why our healthcare costs are the highest in the world," said Feld. "There's no detection test for ALS. No brain detection for Alzheimer's. Innovations in detection technology would save on cost and human suffering."
Some biotech companies may be skeptical about the financial rewards of accelerating such technologies. But ARPA-H could fund public-private partnerships to "de-risk" biotech's involvement—an incentive that harkens back to the advance purchase contracts that companies got during Covid. (Some groups have suggested that ARPA-H could provide advance purchase agreements.)
Parikh is less bullish on creating diagnostics through ARPA-H. Like DARPA, Biden's health agency will enjoy some independence from federal oversight; it may even be located hundreds of miles from DC. That freedom affords some breathing room for innovation, but it could also make it tougher to ensure that algorithms fully consider diverse populations. "That part I really would like the government more involved in," Parikh said.
Might thinks ARPA-H should also explore innovations in clinical trials, which many patients and medical communities view as grindingly slow and requiring too many participants. "We can approve drugs for very tiny patient populations, even at the level of the individual," he said, while emphasizing the need for safety. But Platanias thinks the FDA has become much more flexible in recent years. In the cancer field, at least, "You now see faster approvals for more drugs. Having [more] shortcuts on clinical trial approvals is not necessarily a good idea."
With so many options on the table, ARPA-H needs to show the public a clear framework for measuring the value of potential projects. Kass warned that well-resourced advocates could skew the agency's priorities. They've affected health outcomes before, she noted; fundraising may partly explain larger increases in life expectancy for cystic fibrosis than sickle cell anemia. Engaging diverse communities is a must for ARPA-H. So are partnerships to get the agency's outputs to people who need them. "Research is half the equation," said Kass. "If we don't ensure implementation and access, who cares." The White House concept paper on ARPA-H made a similar point.
As Congress works on authorizing ARPA-H this year, Might is doing what he can to ensure better access to innovation on a patient-by-patient basis. Last year, his son, Bertrand, passed away suddenly from his disorder. He was 12. But Might's sense of urgency has persisted, as he directs the Precision Medicine Institute at the University of Alabama-Birmingham. That urgency "can be carried into an agency like ARPA-H," he said. "It guides what I do as I apply for funding, because I'm trying to build the infrastructure that other parents need. So they don't have to build it from scratch like I did."
Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
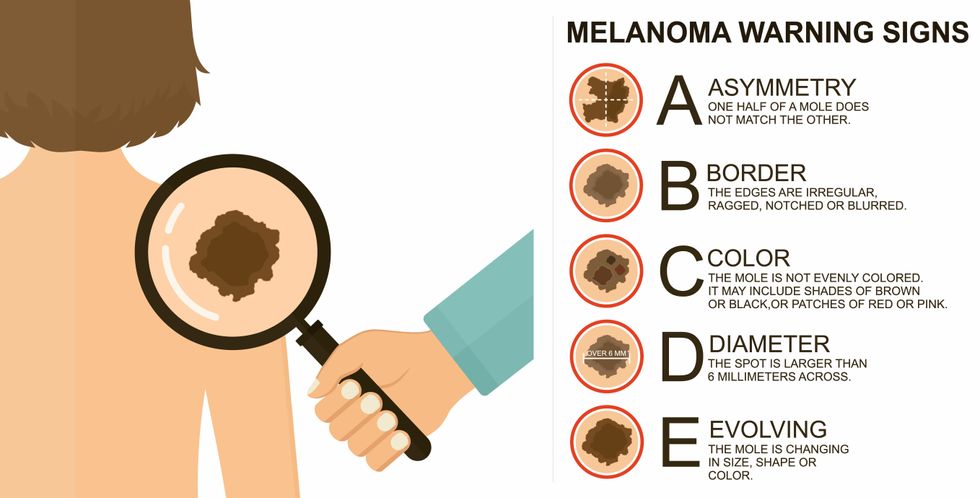
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.

