The Voice Behind Some of Your Favorite Cartoon Characters Helped Create the Artificial Heart

This Jarvik-7 artificial heart was used in the first bridge operation in 1985 meant to replace a failing heart while the patient waited for a donor organ.
In June, a team of surgeons at Duke University Hospital implanted the latest model of an artificial heart in a 39-year-old man with severe heart failure, a condition in which the heart doesn't pump properly. The man's mechanical heart, made by French company Carmat, is a new generation artificial heart and the first of its kind to be transplanted in the United States. It connects to a portable external power supply and is designed to keep the patient alive until a replacement organ becomes available.
Many patients die while waiting for a heart transplant, but artificial hearts can bridge the gap. Though not a permanent solution for heart failure, artificial hearts have saved countless lives since their first implantation in 1982.
What might surprise you is that the origin of the artificial heart dates back decades before, when an inventive television actor teamed up with a famous doctor to design and patent the first such device.
A man of many talents
Paul Winchell was an entertainer in the 1950s and 60s, rising to fame as a ventriloquist and guest-starring as an actor on programs like "The Ed Sullivan Show" and "Perry Mason." When children's animation boomed in the 1960s, Winchell made a name for himself as a voice actor on shows like "The Smurfs," "Winnie the Pooh," and "The Jetsons." He eventually became famous for originating the voices of Tigger from "Winnie the Pooh" and Gargamel from "The Smurfs," among many others.
But Winchell wasn't just an entertainer: He also had a quiet passion for science and medicine. Between television gigs, Winchell busied himself working as a medical hypnotist and acupuncturist, treating the same Hollywood stars he performed alongside. When he wasn't doing that, Winchell threw himself into engineering and design, building not only the ventriloquism dummies he used on his television appearances but a host of products he'd dreamed up himself. Winchell spent hours tinkering with his own inventions, such as a set of battery-powered gloves and something called a "flameless lighter." Over the course of his life, Winchell designed and patented more than 30 of these products – mostly novelties, but also serious medical devices, such as a portable blood plasma defroster.

| Ventriloquist Paul Winchell with Jerry Mahoney, his dummy, in 1951 |
A meeting of the minds
In the early 1950s, Winchell appeared on a variety show called the "Arthur Murray Dance Party" and faced off in a dance competition with the legendary Ricardo Montalban (Winchell won). At a cast party for the show later that same night, Winchell met Dr. Henry Heimlich – the same doctor who would later become famous for inventing the Heimlich maneuver, who was married to Murray's daughter. The two hit it off immediately, bonding over their shared interest in medicine. Before long, Heimlich invited Winchell to come observe him in the operating room at the hospital where he worked. Winchell jumped at the opportunity, and not long after he became a frequent guest in Heimlich's surgical theatre, fascinated by the mechanics of the human body.
One day while Winchell was observing at the hospital, he witnessed a patient die on the operating table after undergoing open-heart surgery. He was suddenly struck with an idea: If there was some way doctors could keep blood pumping temporarily throughout the body during surgery, patients who underwent risky operations like open-heart surgery might have a better chance of survival. Winchell rushed to Heimlich with the idea – and Heimlich agreed to advise Winchell and look over any design drafts he came up with. So Winchell went to work.
Winchell's heart
As it turned out, building ventriloquism dummies wasn't that different from building an artificial heart, Winchell noted later in his autobiography – the shifting valves and chambers of the mechanical heart were similar to the moving eyes and opening mouths of his puppets. After each design, Winchell would go back to Heimlich and the two would confer, making adjustments along the way to.
By 1956, Winchell had perfected his design: The "heart" consisted of a bag that could be placed inside the human body, connected to a battery-powered motor outside of the body. The motor enabled the bag to pump blood throughout the body, similar to a real human heart. Winchell received a patent for the design in 1963.
At the time, Winchell never quite got the credit he deserved. Years later, researchers at the University of Utah, working on their own artificial heart, came across Winchell's patent and got in touch with Winchell to compare notes. Winchell ended up donating his patent to the team, which included Dr. Richard Jarvik. Jarvik expanded on Winchell's design and created the Jarvik-7 – the world's first artificial heart to be successfully implanted in a human being in 1982.
The Jarvik-7 has since been replaced with newer, more efficient models made up of different synthetic materials, allowing patients to live for longer stretches without the heart clogging or breaking down. With each new generation of hearts, heart failure patients have been able to live relatively normal lives for longer periods of time and with fewer complications than before – and it never would have been possible without the unsung genius of a puppeteer and his love of science.
For Kids with Progeria, New Therapies May Offer Revolutionary Hope for a Longer Life
Sammy Basso is one of around 400 young people in the world with progeria.
Sammy Basso has some profound ideas about fate. As long as he has been alive, he has known he has minimal control over his own. His parents, however, had to transition from a world of unlimited possibility to one in which their son might not live to his 20s.
"I remember very clearly that day because Sammy was three years old," his mother says of the day a genetic counselor diagnosed Sammy with progeria. "It was a devastating day for me."
But to Sammy, he has always been himself: a smart kid, interested in science, a little smaller than his classmates, with one notable kink in his DNA. In one copy of the gene that codes for the protein Lamin A, Sammy has a T where there should be a C. The incorrect code creates a toxic protein called progerin, which destabilizes Sammy's cells and makes him age much faster than a person who doesn't have the mutation. The older he gets, the more he is in danger of strokes, heart failure, or a heart attack. "I am okay with my situation," he says from his home in Tezze sul Brenta, Italy. "But I think, yes, fate has a great role in my life."
Just 400 or so people in the world live with progeria: The mutation that causes it usually arises de novo, or "of new," meaning that it is not inherited but happens spontaneously during gestation. The challenge, as with all rare diseases, is that few cases means few treatments.
"When we first started, there was absolutely nothing out there," says Leslie Gordon, a physician-researcher who co-founded the Progeria Research Foundation in 1999 after her own son, also named Sam, was diagnosed with the disease. "We knew we had to jumpstart the entire field, so we collected money through road races and special events and writing grants and all sorts of donors… I think the first year we raised $75,000, most of it from one donor."
"We have not only the possibility but the responsibility to make the world a better world, and also to make a body a better body."
By 2003, the foundation had collaborated with Francis Collins, a geneticist who is now director of the National Institutes of Health, to work out the genetic basis for progeria—that single mutation Sammy has. The discovery led to interest in lonafarnib, a drug that was already being used in cancer patients but could potentially operate downstream of the mutation, preventing the buildup of the defective progerin in the body. "We funded cellular studies to look at a lonafarnib in cells, mouse studies to look at lonafarnib in mouse models of progeria… and then we initiated the clinical trials," Gordon says.
Sammy Basso's family had gotten involved with the Progeria Research Foundation through their international patient registry, which maintains relationships with families in 49 countries. "We started to hear about lonafarnib in 2006 from Leslie Gordon," says Sammy's father, Amerigo Basso, with his son translating. "She told us about the lonafarnib. And we were very happy because for the first time we understood that there was something that could help our son and our lives." Amerigo used the Italian word speranza, which means hope.
Still, Sammy wasn't sure if lonafarnib was right for him. "Since when I was very young I thought that everything happens for a reason. So, in my mind, if God made me with progeria, there was a reason, and to try to heal from progeria was something wrong," he says. Gradually, his parents and doctors, and Leslie Gordon, convinced him otherwise. Sammy began to believe that God was also the force behind doctors, science, and research. "And so we have not only the possibility but the responsibility to make the world a better world, and also to make a body a better body," he says.

Sammy Basso and his parents.
Courtesy of Basso
Sammy began taking lonafarnib, with the Progeria Research Foundation intermittently flying him, and other international trial participants, to Boston for tests. He was immediately beset by some of the drug's more unpleasant side effects: Stomach problems, nausea, and vomiting. "The first period was absolutely the worst period of my life," he says.
At first, doctors prescribed other medicines for the side effects, but to Sammy it had as much effect as drinking water. He visited doctor after doctor, with some calling him weekly or even daily to ask how he was doing. Eventually the specialists decided that he should lower his dose, balancing his pain with the benefit of the drug. Sammy can't actually feel any positive effect of the lonafarnib, but his health measurements have improved relative to people with progeria who don't take it.
While they never completely disappeared, Sammy's side effects decreased to the point that he could live. Inspired by the research that led to lonafarnib, he went to university to study molecular biology. For his thesis work, he travelled to Spain to perform experiments on cells and on mice with progeria, learning how to use the gene-editing technique CRISPR-Cas9 to cut out the mutated bit of DNA. "I was so excited to participate in this study," Sammy says. He felt like his work could make a difference.
In 2018, the Progeria Research Foundation was hosting one of their biennial workshops when Francis Collins, the researcher who had located the mutation behind progeria 15 years earlier, got in touch with Leslie Gordon. "Francis called me and said, Hey, I just saw a talk by David Liu from the Broad [Institute]. And it was pretty amazing. He has been looking at progeria and has very early, but very exciting data… Do you have any spaces, any slots you could make in your program for late breaking news?"
Gordon found a spot, and David Liu came to talk about what was going on in his lab, which was an even more advanced treatment that led to mice with the progeria mutation living into their senior mouse years—substantially closer to a normal lifespan. Liu's lab had built on the idea of CRISPR-Cas9 to create a more elegant genetic process called base editing: Instead of chopping out mutated DNA, a scientist could chemically convert an incorrect DNA letter to the correct one, like the search and replace function in word processing software. Mice who had their Lamin-A mutations corrected this way lived more than twice as long as untreated animals.
Sammy was in the audience at Dr. Liu's talk. "When I heard about this base editing as a younger scientist, I thought that I was living in the future," he says. "When my parents had my diagnosis of progeria, the science knew very little information about DNA. And now we are talking about healing the DNA… It is incredible."
Lonafarnib (also called Zokinvy) was approved by the US Food and Drug Administration this past November. Sammy, now 25, still takes it, and still manages his side effects. With luck, the gift of a few extra years will act as a bridge until he can try Liu's revolutionary new gene treatment, which has not yet begun testing in humans. While Leslie Gordon warns that she's always wrong about things like this, she hopes to see the new base editing techniques in clinical trials in the next year or two. Sammy won't need to be convinced to try it this time; his thinking on fate has evolved since his first encounter with lonafarnib.
"I would be very happy to try it," he says. "I know that for a non-scientist it can be difficult to understand. Some people think that we are the DNA. We are not. The DNA is a part of us, and to correct it is to do what we are already doing—just better." In short, a gene therapy, while it may seem like science fiction, is no different from a pill. For Sammy, both are a new way to think about fate: No longer something that simply happens to him.
Want to Motivate Vaccinations? Message Optimism, Not Doom
There is a lot to be optimistic about regarding the new safe and highly effective vaccines, which are moving society closer toward the goal of close human contact once again.
After COVID-19 was declared a worldwide pandemic by the World Health Organization on March 11, 2020, life as we knew it altered dramatically and millions went into lockdown. Since then, most of the world has had to contend with masks, distancing, ventilation and cycles of lockdowns as surges flare up. Deaths from COVID-19 infection, along with economic and mental health effects from the shutdowns, have been devastating. The need for an ultimate solution -- safe and effective vaccines -- has been paramount.
On November 9, 2020 (just 8 months after the pandemic announcement), the press release for the first effective COVID-19 vaccine from Pfizer/BioNTech was issued, followed by positive announcements regarding the safety and efficacy of five other vaccines from Moderna, University of Oxford/AztraZeneca, Novavax, Johnson and Johnson and Sputnik V. The Moderna and Pfizer vaccines have earned emergency use authorization through the FDA in the United States and are being distributed. We -- after many long months -- are seeing control of the devastating COVID-19 pandemic glimmering into sight.
To be clear, these vaccine candidates for COVID-19, both authorized and not yet authorized, are highly effective and safe. In fact, across all trials and sites, all six vaccines were 100% effective in preventing hospitalizations and death from COVID-19.
All Vaccines' Phase 3 Clinical Data
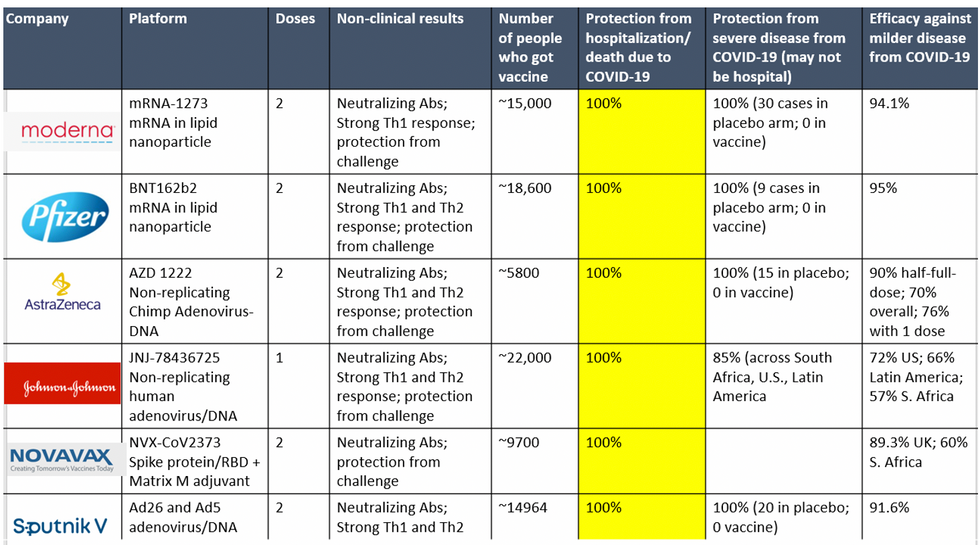
Complete protection against hospitalization and death from COVID-19 exhibited by all vaccines with phase 3 clinical trial data.
This astounding level of protection from SARS-CoV-2 from all vaccine candidates across multiple regions is likely due to robust T cell response from vaccination and will "defang" the virus from the concerns that led to COVID-19 restrictions initially: the ability of the virus to cause severe illness. This is a time of hope and optimism. After the devastating third surge of COVID-19 infections and deaths over the winter, we finally have an opportunity to stem the crisis – if only people readily accept the vaccines.
Amidst these incredible scientific advancements, however, public health officials and politicians have been pushing downright discouraging messaging. The ubiquitous talk of ongoing masks and distancing restrictions without any clear end in sight threatens to dampen uptake of the vaccines. It's imperative that we break down each concern and see if we can revitalize our public health messaging accordingly.
The first concern: we currently do not know if the vaccines block asymptomatic infection as well as symptomatic disease, since none of the phase 3 vaccine trials were set up to answer this question. However, there is biological plausibility that the antibodies and T-cell responses blocking symptomatic disease will also block asymptomatic infection in the nasal passages. IgG immunoglobulins (generated and measured by the vaccine trials) enter the nasal mucosa and systemic vaccinations generate IgA antibodies at mucosal surfaces. Monoclonal antibodies given to outpatients with COVID-19 hasten viral clearance from the airways.
Although it is prudent for those who are vaccinated to wear masks around the unvaccinated in case a slight risk of transmission remains, two fully vaccinated people can comfortably abandon masking around each other.
Moreover, data from the AztraZeneca trial (including in the phase 3 trial final results manuscript), where weekly self-swabbing was done by participants, and data from the Moderna trial, where a nasal swab was performed prior to the second dose, both showed risk reductions in asymptomatic infection with even a single dose. Finally, real-world data from a large Pfizer-based vaccine campaign in Israel shows a 50% reduction in infections (asymptomatic or symptomatic) after just the first dose.
Therefore, the likelihood of these vaccines blocking asymptomatic carriage, as well as symptomatic disease, is high. Although it is prudent for those who are vaccinated to wear masks around the unvaccinated in case a slight risk of transmission remains, two fully vaccinated people can comfortably abandon masking around each other. Moreover, as the percentage of vaccinated people increases, it will be increasingly untenable to impose restrictions on this group. Once herd immunity is reached, these restrictions can and should be abandoned altogether.
The second concern translating to "doom and gloom" messaging lately is around the identification of troubling new variants due to enhanced surveillance via viral sequencing. Four major variants circulating at this point (with others described in the past) are the B.1.1.7 variant ("UK variant"), B.1.351 ("South Africa variant), P.1. ("Brazil variant"), and the L452R variant identified in California. Although the UK variant is likely to be more transmissible, as is the South Africa variant, we have no reason to believe that masks, distancing and ventilation are ineffective against these variants.
Moreover, neutralizing antibody titers with the Pfizer and Moderna vaccines do not seem to be significantly reduced against the variants. Finally, although the Novavax 2-dose and Johnson and Johnson (J&J) 1-dose vaccines had lower rates of efficacy against moderate COVID-19 disease in South Africa, their efficacy against severe disease was impressively high. In fact J&J's vaccine still prevented 100% of hospitalizations and death from COVID-19. When combining both hospitalizations/deaths and severe symptoms managed at home, the J&J 1-dose vaccine was 85% protective across all three sites of the trial: the U.S., Latin America (including Brazil), and South Africa.
In South Africa, nearly all cases of COVID-19 (95%) were due to infection with the B.1.351 SARS-CoV-2 variant. Finally, since herd immunity does not rely on maximal immune responses among all individuals in a society, the Moderna/Pfizer/J&J vaccines are all likely to achieve that goal against variants. And thankfully, all of these vaccines can be easily modified to boost specifically against a new variant if needed (indeed, Moderna and Pfizer are already working on boosters against the prominent variants).
The third concern of some public health officials is that people will abandon all restrictions once vaccinated unless overly cautious messages are drilled into them. Indeed, the false idea that if you "give people an inch, they will take a mile" has been misinforming our messaging about mitigation since the beginning of the pandemic. For example, the very phrase "stay at home" with all of its non-applicability for essential workers and single individuals is stigmatizing and unrealistic for many. Instead, the message should have focused on how people can additively reduce their risks under different circumstances.
The public will be more inclined to trust health officials if those officials communicate with nuanced messages backed up by evidence, rather than with broad brushstrokes that shame. Therefore, we should be saying that "vaccinated people can be together with other vaccinated individuals without restrictions but must protect the unvaccinated with masks and distancing." And we can say "unvaccinated individuals should adhere to all current restrictions until vaccinated" without fear of misunderstandings. Indeed, this kind of layered advice has been communicated to people living with HIV and those without HIV for a long time (if you have HIV but partner does not, take these precautions; if both have HIV, you can do this, etc.).
Our heady progress in vaccine development, along with the incredible efficacy results of all of them, is unprecedented. However, we are at risk of undermining such progress if people balk at the vaccine because they don't believe it will make enough of a difference. One of the most critical messages we can deliver right now is that these vaccines will eventually free us from the restrictions of this pandemic. Let's use tiered messaging and clear communication to boost vaccine optimism and uptake, and get us to the goal of close human contact once again.