Artificial Wombs Are Getting Closer to Reality for Premature Babies
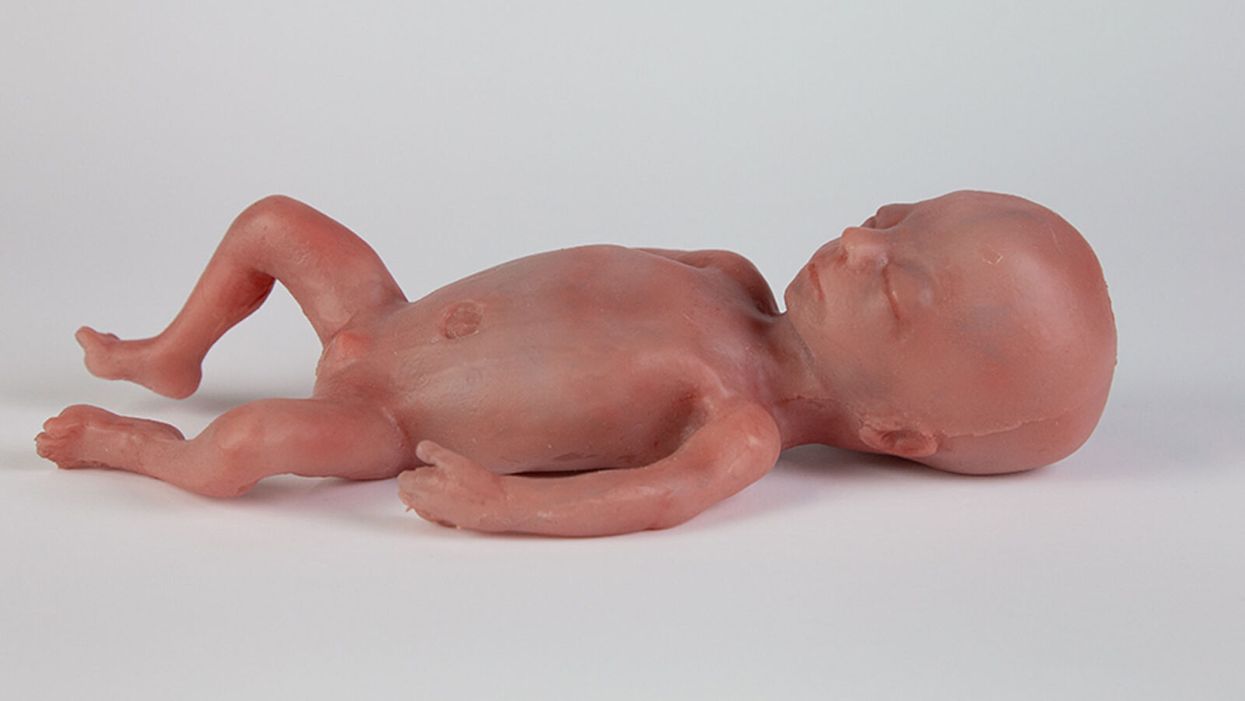
A mannequin of a 24-week-old fetus replicated from MR imaging. Created by: Juliette van Haren, Mark Thielen, Jasper Sterk, Chet Bangaru, and Frank Delbressine, Department of Industrial Design, Eindhoven University of Technology.
In 2017, researchers at the Children's Hospital of Philadelphia grew extremely preterm lambs from hairless to fluffy inside a "biobag," a dark, fluid-filled bag designed to mimic a mother's womb.
"There could be quite a lot of infants that would benefit from artificial womb technologies."
This happened over the course of a month, across a delicate period of fetal development that scientists consider the "edge of viability" for survival at birth.
In 2019, Australian and Japanese scientists repeated the success of keeping extremely premature lambs inside an artificial womb environment until they were ready to survive on their own. Those researchers are now developing a treatment strategy for infants born at "the hard limit of viability," between 20 and 23 weeks of gestation. At the same time, Dutch researchers are going so far as to replicate the sound of a mother's heartbeat inside a biobag. These developments signal exciting times ahead--with a touch of science fiction--for artificial womb technologies. But is there a catch?
"There could be quite a lot of infants that would benefit from artificial womb technologies," says Josephine Johnston, a bioethicist and lawyer at The Hastings Center, an independent bioethics research institute in New York. "These technologies can decrease morbidity and mortality for infants at the edge of viability and help them survive without significant damage to the lungs or other problems," she says.
It is a viewpoint shared by Frans van de Vosse, leader of the Cardiovascular Biomechanics research group at Eindhoven University of Technology in the Netherlands. He participates in a university project that recently received more than $3 million in funding from the E.U. to produce a prototype artificial womb for preterm babies between 24 and 28 weeks of gestation by 2024.
The Eindhoven design comes with a fluid-based environment, just like that of the natural womb, where the baby receives oxygen and nutrients through an artificial placenta that is connected to the baby's umbilical cord. "With current incubators, when a respiratory device delivers oxygen into the lungs in order for the baby to breathe, you may harm preterm babies because their lungs are not yet mature for that," says van de Vosse. "But when the lungs are under water, then they can develop, they can mature, and the baby will receive the oxygen through the umbilical cord, just like in the natural womb," he says.
His research team is working to achieve the "perfectly natural" artificial womb based on strict mathematical models and calculations, van de Vosse says. They are even employing 3D printing technology to develop the wombs and artificial babies to test in them--the mannequins, as van de Vosse calls them. These mannequins are being outfitted with sensors that can replicate the environment a fetus experiences inside a mother's womb, including the soothing sound of her heartbeat.
"The Dutch study's artificial womb design is slightly different from everything else we have seen as it encourages a gestateling to experience the kind of intimacy that a fetus does in pregnancy," says Elizabeth Chloe Romanis, an assistant professor in biolaw at Durham Law School in the U.K. But what is a "gestateling" anyway? It's a term Romanis has coined to describe neither a fetus nor a newborn, but an in-between artificial stage.
"Because they aren't born, they are not neonates," Romanis explains. "But also, they are not inside a pregnant person's body, so they are not fetuses. In an artificial womb the fetus is still gestating, hence why I call it gestateling."
The terminology is not just a semantic exercise to lend a name to what medical dictionaries haven't yet defined. "Gestatelings might have a slightly different psychology," says Romanis. "A fetus inside a mother's womb interacts with the mother. A neonate has some kind of self-sufficiency in terms of physiology. But the gestateling doesn't do either of those things," she says, urging us to be mindful of the still-obscure effects that experiencing early life as a gestateling might have on future humans. Psychology aside, there are also legal repercussions.
The Universal Declaration of Human Rights proclaims the "inalienable rights which everyone is entitled to as a human being," with "everyone" including neonates. However, such a legal umbrella is absent when it comes to fetuses, which have no rights under the same declaration. "We might need a new legal category for a gestateling," concludes Romanis.
But not everyone agrees. "However well-meaning, a new legal category would almost certainly be used to further erode the legality of abortion in countries like the U.S.," says Johnston.
The "abortion war" in the U.S. has risen to a crescendo since 2019, when states like Missouri, Mississippi, Kentucky, Louisiana and Georgia passed so-called "fetal heartbeat bills," which render an abortion illegal once a fetal heartbeat is detected. The situation is only bound to intensify now that Justice Ruth Bader Ginsburg, one of the Supreme Court's fiercest champions for abortion rights, has passed away. If President Trump appoints Ginsburg's replacement, he will probably grant conservatives on the Court the votes needed to revoke or weaken Roe v. Wade, the milestone decision of 1973 that established women's legal right to an abortion.
"A gestateling with intermediate status would almost certainly be considered by some in the U.S. (including some judges) to have at least certain legal rights, likely including right-to-life," says Johnston. This would enable a fetus on the edge of viability to make claims on the mother, and lead either to a shortening of the window in which abortion is legal—or a practice of denying abortion altogether. Instead, Johnston predicts, doctors might offer to transfer the fetus to an artificial womb for external gestation as a new standard of care.
But the legal conundrum does not stop there. The viability threshold is an estimate decided by medical professionals based on the clinical evidence and the technology available. It is anything but static. In the 1970s when Roe v. Wade was decided, for example, a fetus was considered legally viable starting at 28 weeks. Now, with improved technology and medical management, "the hard limit today is probably 20 or 21 weeks," says Matthew Kemp, associate professor at the University of Western Australia and one of the Australian-Japanese artificial womb project's senior researchers.
The changing threshold can result in situations where lots of people invested in the decision disagree. "Those can be hard decisions, but they are case-by-case decisions that families make or parents make with the key providers to determine when to proceed and when to let the infant die. Usually, it's a shared decision where the parents have the final say," says Johnston. But this isn't always the case.
On May 9th 2016, a boy named Alfie Evans was born in Liverpool, UK. Suffering seizures a few months after his birth, Alfie was diagnosed with an unknown neurodegenerative disorder and soon went into a semi-vegetative state, which lasted for more than a year. Alfie's medical team decided to withdraw his ventilation support, suggesting further treatment was unlawful and inhumane, but his parents wanted permission to fly him to a hospital in Rome and attempt to prolong his life there. In the end, the case went all the way up to the Supreme Court, which ruled that doctors could stop providing life support for Alfie, saying that the child required "peace, quiet and privacy." What happened to little Alfie raised huge publicity in the UK and pointedly highlighted the dilemma of whether parents or doctors should have the final say in the fate of a terminally-ill child in life-support treatment.
"In a few years from now, women who cannot get pregnant because of uterine infertility will be able to have a fully functional uterus made from their own tissue."
Alfie was born and, thus had legal rights, yet legal and ethical mayhem arose out of his case. When it comes to gestatelings, the scenarios will be even more complicated, says Romanis. "I think there's a really big question about who has parental rights and who doesn't," she says. "The assisted reproductive technology (ART) law in the U.K. hasn't been updated since 2008....It certainly needs an update when you think about all the things we have done since [then]."
This June, for instance, scientists from the Wake Forest Institute for Regenerative Medicine in North Carolina published research showing that they could take a small sample of tissue from a rabbit's uterus and create a bioengineered uterus, which then supported both fertilization and normal pregnancy like a natural uterus does.
"In [a number of] years from now, women who cannot get pregnant because of uterine infertility will be able to have a fully functional uterus made from their own tissue," says Dr. Anthony Atala, the Institute's director and a pioneer in regenerative medicine. These bioengineered uteri will eventually be covered by insurance, Atala expects. But when it comes to artificial wombs that externally gestate premature infants, will all mothers have equal access?
Medical reports have already shown racial and ethnic disparities in infertility treatments and access to assisted reproductive technologies. Costs on average total $12,400 per cycle of treatment and may require several cycles to achieve a live birth. "There's no indication that artificial wombs would be treated any differently. That's what we see with almost every expensive new medical technology," says Johnston. In a much more dystopian future, there is even a possibility that inequity in healthcare might create disturbing chasms in how women of various class levels bear children. Romanis asks us to picture the following scenario:
We live in a world where artificial wombs have become mainstream. Most women choose to end their pregnancies early and transfer their gestatelings to the care of machines. After a while, insurers deem full-term pregnancy and childbirth a risky non-necessity, and are lobbying to stop covering them altogether. Wealthy white women continue opting out of their third trimesters (at a high cost), since natural pregnancy has become a substandard route for poorer women. Those women are strongly judged for any behaviors that could risk their fetus's health, in contrast with the machine's controlled environment. "Why are you having a coffee during your pregnancy?" critics might ask. "Why are you having a glass of red wine? If you can't be perfect, why don't you have it the artificial way?"
Problem is, even if they want to, they won't be able to afford it.
In a more sanguine version, however, the artificial wombs are only used in cases of prematurity as a life-saving medical intervention rather than as a lifestyle accommodation. The 15 million babies who are born prematurely each year and may face serious respiratory, cardiovascular, visual and hearing problems, as well as learning disabilities, instead continue their normal development in artificial wombs. After lots of deliberation, insurers agree to bear the cost of external wombs because they are cheaper than a lifetime of medical care for a disabled or diseased person. This enables racial and ethnic minority women, who make up the majority of women giving premature birth, to access the technology.
Even extremely premature babies, those babies (far) below the threshold of 28 weeks of gestation, half of which die, could now discover this thing called life. In this scenario, as the Australian researcher Kemp says, we are simply giving a good shot at healthy, long-term survival to those who were unfortunate enough to start too soon.
Stronger psychedelics that rewire the brain, with Doug Drysdale
Today's podcast episode features Doug Drysdale, CEO of Cybin, a company that is leading innovations in psilocybin, mushrooms that may help people with anxiety and depression.
A promising development in science in recent years has been the use technology to optimize something natural. One-upping nature's wisdom isn't easy. In many cases, we haven't - and maybe we can't - figure it out. But today's episode features a fascinating example: using tech to optimize psychedelic mushrooms.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
These mushrooms have been used for religious, spiritual and medicinal purposes for thousands of years, but only in the past several decades have scientists brought psychedelics into the lab to enhance them and maximize their therapeutic value.
Today’s podcast guest, Doug Drysdale, is doing important work to lead this effort. Drysdale is the CEO of a company called Cybin that has figured out how to make psilocybin more potent, so it can be administered in smaller doses without side effects.
The natural form of psilocybin has been studied increasingly in the realm of mental health. Taking doses of these mushrooms appears to help people with anxiety and depression by spurring the development of connections in the brain, an example of neuroplasticity. The process basically shifts the adult brain from being fairly rigid like dried clay into a malleable substance like warm wax - the state of change that's constantly underway in the developing brains of children.
Neuroplasticity in adults seems to unlock some of our default ways of of thinking, the habitual thought patterns that’ve been associated with various mental health problems. Some promising research suggests that psilocybin causes a reset of sorts. It makes way for new, healthier thought patterns.
So what is Drysdale’s secret weapon to bring even more therapeutic value to psilocybin? It’s a process called deuteration. It focuses on the hydrogen atoms in psilocybin. These atoms are very light and don’t stick very well to carbon, which is another atom in psilocybin. As a result, our bodies can easily breaks down the bonds between the hydrogen and carbon atoms. For many people, that means psilocybin gets cleared from the body too quickly, before it can have a therapeutic benefit.
In deuteration, scientists do something simple but ingenious: they replace the hydrogen atoms with a molecule called deuterium. It’s twice as heavy as hydrogen and forms tighter bonds with the carbon. Because these pairs are so rock-steady, they slow down the rate at which psilocybin is metabolized, so it has more sustained effects on our brains.
Cybin isn’t Drysdale’s first go around at this - far from it. He has over 30 years of experience in the healthcare sector. During this time he’s raised around $4 billion of both public and private capital, and has been named Ernst and Young Entrepreneur of the Year. Before Cybin, he was the founding CEO of a pharmaceutical company called Alvogen, leading it from inception to around $500 million in revenues, across 35 countries. Drysdale has also been the head of mergers and acquisitions at Actavis Group, leading 15 corporate acquisitions across three continents.
In this episode, Drysdale walks us through the promising research of his current company, Cybin, and the different therapies he’s developing for anxiety and depression based not just on psilocybin but another psychedelic compound found in plants called DMT. He explains how they seem to have such powerful effects on the brain, as well as the potential for psychedelics to eventually support other use cases, including helping us strive toward higher levels of well-being. He goes on to discuss his views on mindfulness and lifestyle factors - such as optimal nutrition - that could help bring out hte best in psychedelics.
Show links:
Doug Drysdale full bio
Doug Drysdale twitter
Cybin website
Cybin development pipeline
Cybin's promising phase 2 research on depression
Johns Hopkins psychedelics research and psilocybin research
Mets owner Steve Cohen invests in psychedelic therapies

Doug Drysdale, CEO of Cybin
How the body's immune resilience affects our health and lifespan
Immune cells battle an infection.
Story by Big Think
It is a mystery why humans manifest vast differences in lifespan, health, and susceptibility to infectious diseases. However, a team of international scientists has revealed that the capacity to resist or recover from infections and inflammation (a trait they call “immune resilience”) is one of the major contributors to these differences.
Immune resilience involves controlling inflammation and preserving or rapidly restoring immune activity at any age, explained Weijing He, a study co-author. He and his colleagues discovered that people with the highest level of immune resilience were more likely to live longer, resist infection and recurrence of skin cancer, and survive COVID and sepsis.
Measuring immune resilience
The researchers measured immune resilience in two ways. The first is based on the relative quantities of two types of immune cells, CD4+ T cells and CD8+ T cells. CD4+ T cells coordinate the immune system’s response to pathogens and are often used to measure immune health (with higher levels typically suggesting a stronger immune system). However, in 2021, the researchers found that a low level of CD8+ T cells (which are responsible for killing damaged or infected cells) is also an important indicator of immune health. In fact, patients with high levels of CD4+ T cells and low levels of CD8+ T cells during SARS-CoV-2 and HIV infection were the least likely to develop severe COVID and AIDS.
Individuals with optimal levels of immune resilience were more likely to live longer.
In the same 2021 study, the researchers identified a second measure of immune resilience that involves two gene expression signatures correlated with an infected person’s risk of death. One of the signatures was linked to a higher risk of death; it includes genes related to inflammation — an essential process for jumpstarting the immune system but one that can cause considerable damage if left unbridled. The other signature was linked to a greater chance of survival; it includes genes related to keeping inflammation in check. These genes help the immune system mount a balanced immune response during infection and taper down the response after the threat is gone. The researchers found that participants who expressed the optimal combination of genes lived longer.
Immune resilience and longevity
The researchers assessed levels of immune resilience in nearly 50,000 participants of different ages and with various types of challenges to their immune systems, including acute infections, chronic diseases, and cancers. Their evaluation demonstrated that individuals with optimal levels of immune resilience were more likely to live longer, resist HIV and influenza infections, resist recurrence of skin cancer after kidney transplant, survive COVID infection, and survive sepsis.
However, a person’s immune resilience fluctuates all the time. Study participants who had optimal immune resilience before common symptomatic viral infections like a cold or the flu experienced a shift in their gene expression to poor immune resilience within 48 hours of symptom onset. As these people recovered from their infection, many gradually returned to the more favorable gene expression levels they had before. However, nearly 30% who once had optimal immune resilience did not fully regain that survival-associated profile by the end of the cold and flu season, even though they had recovered from their illness.
Intriguingly, some people who are 90+ years old still have optimal immune resilience, suggesting that these individuals’ immune systems have an exceptional capacity to control inflammation and rapidly restore proper immune balance.
This could suggest that the recovery phase varies among people and diseases. For example, young female sex workers who had many clients and did not use condoms — and thus were repeatedly exposed to sexually transmitted pathogens — had very low immune resilience. However, most of the sex workers who began reducing their exposure to sexually transmitted pathogens by using condoms and decreasing their number of sex partners experienced an improvement in immune resilience over the next 10 years.
Immune resilience and aging
The researchers found that the proportion of people with optimal immune resilience tended to be highest among the young and lowest among the elderly. The researchers suggest that, as people age, they are exposed to increasingly more health conditions (acute infections, chronic diseases, cancers, etc.) which challenge their immune systems to undergo a “respond-and-recover” cycle. During the response phase, CD8+ T cells and inflammatory gene expression increase, and during the recovery phase, they go back down.
However, over a lifetime of repeated challenges, the immune system is slower to recover, altering a person’s immune resilience. Intriguingly, some people who are 90+ years old still have optimal immune resilience, suggesting that these individuals’ immune systems have an exceptional capacity to control inflammation and rapidly restore proper immune balance despite the many respond-and-recover cycles that their immune systems have faced.
Public health ramifications could be significant. Immune cell and gene expression profile assessments are relatively simple to conduct, and being able to determine a person’s immune resilience can help identify whether someone is at greater risk for developing diseases, how they will respond to treatment, and whether, as well as to what extent, they will recover.