Artificial Wombs Are Getting Closer to Reality for Premature Babies
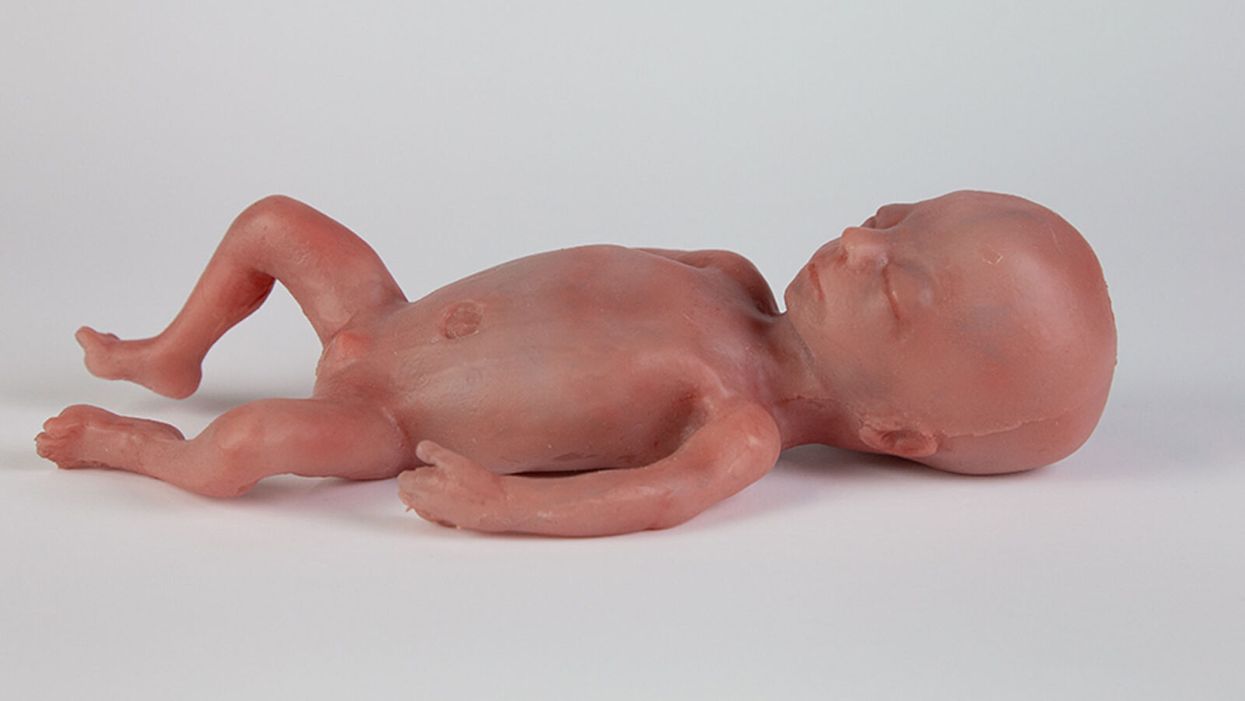
A mannequin of a 24-week-old fetus replicated from MR imaging. Created by: Juliette van Haren, Mark Thielen, Jasper Sterk, Chet Bangaru, and Frank Delbressine, Department of Industrial Design, Eindhoven University of Technology.
In 2017, researchers at the Children's Hospital of Philadelphia grew extremely preterm lambs from hairless to fluffy inside a "biobag," a dark, fluid-filled bag designed to mimic a mother's womb.
"There could be quite a lot of infants that would benefit from artificial womb technologies."
This happened over the course of a month, across a delicate period of fetal development that scientists consider the "edge of viability" for survival at birth.
In 2019, Australian and Japanese scientists repeated the success of keeping extremely premature lambs inside an artificial womb environment until they were ready to survive on their own. Those researchers are now developing a treatment strategy for infants born at "the hard limit of viability," between 20 and 23 weeks of gestation. At the same time, Dutch researchers are going so far as to replicate the sound of a mother's heartbeat inside a biobag. These developments signal exciting times ahead--with a touch of science fiction--for artificial womb technologies. But is there a catch?
"There could be quite a lot of infants that would benefit from artificial womb technologies," says Josephine Johnston, a bioethicist and lawyer at The Hastings Center, an independent bioethics research institute in New York. "These technologies can decrease morbidity and mortality for infants at the edge of viability and help them survive without significant damage to the lungs or other problems," she says.
It is a viewpoint shared by Frans van de Vosse, leader of the Cardiovascular Biomechanics research group at Eindhoven University of Technology in the Netherlands. He participates in a university project that recently received more than $3 million in funding from the E.U. to produce a prototype artificial womb for preterm babies between 24 and 28 weeks of gestation by 2024.
The Eindhoven design comes with a fluid-based environment, just like that of the natural womb, where the baby receives oxygen and nutrients through an artificial placenta that is connected to the baby's umbilical cord. "With current incubators, when a respiratory device delivers oxygen into the lungs in order for the baby to breathe, you may harm preterm babies because their lungs are not yet mature for that," says van de Vosse. "But when the lungs are under water, then they can develop, they can mature, and the baby will receive the oxygen through the umbilical cord, just like in the natural womb," he says.
His research team is working to achieve the "perfectly natural" artificial womb based on strict mathematical models and calculations, van de Vosse says. They are even employing 3D printing technology to develop the wombs and artificial babies to test in them--the mannequins, as van de Vosse calls them. These mannequins are being outfitted with sensors that can replicate the environment a fetus experiences inside a mother's womb, including the soothing sound of her heartbeat.
"The Dutch study's artificial womb design is slightly different from everything else we have seen as it encourages a gestateling to experience the kind of intimacy that a fetus does in pregnancy," says Elizabeth Chloe Romanis, an assistant professor in biolaw at Durham Law School in the U.K. But what is a "gestateling" anyway? It's a term Romanis has coined to describe neither a fetus nor a newborn, but an in-between artificial stage.
"Because they aren't born, they are not neonates," Romanis explains. "But also, they are not inside a pregnant person's body, so they are not fetuses. In an artificial womb the fetus is still gestating, hence why I call it gestateling."
The terminology is not just a semantic exercise to lend a name to what medical dictionaries haven't yet defined. "Gestatelings might have a slightly different psychology," says Romanis. "A fetus inside a mother's womb interacts with the mother. A neonate has some kind of self-sufficiency in terms of physiology. But the gestateling doesn't do either of those things," she says, urging us to be mindful of the still-obscure effects that experiencing early life as a gestateling might have on future humans. Psychology aside, there are also legal repercussions.
The Universal Declaration of Human Rights proclaims the "inalienable rights which everyone is entitled to as a human being," with "everyone" including neonates. However, such a legal umbrella is absent when it comes to fetuses, which have no rights under the same declaration. "We might need a new legal category for a gestateling," concludes Romanis.
But not everyone agrees. "However well-meaning, a new legal category would almost certainly be used to further erode the legality of abortion in countries like the U.S.," says Johnston.
The "abortion war" in the U.S. has risen to a crescendo since 2019, when states like Missouri, Mississippi, Kentucky, Louisiana and Georgia passed so-called "fetal heartbeat bills," which render an abortion illegal once a fetal heartbeat is detected. The situation is only bound to intensify now that Justice Ruth Bader Ginsburg, one of the Supreme Court's fiercest champions for abortion rights, has passed away. If President Trump appoints Ginsburg's replacement, he will probably grant conservatives on the Court the votes needed to revoke or weaken Roe v. Wade, the milestone decision of 1973 that established women's legal right to an abortion.
"A gestateling with intermediate status would almost certainly be considered by some in the U.S. (including some judges) to have at least certain legal rights, likely including right-to-life," says Johnston. This would enable a fetus on the edge of viability to make claims on the mother, and lead either to a shortening of the window in which abortion is legal—or a practice of denying abortion altogether. Instead, Johnston predicts, doctors might offer to transfer the fetus to an artificial womb for external gestation as a new standard of care.
But the legal conundrum does not stop there. The viability threshold is an estimate decided by medical professionals based on the clinical evidence and the technology available. It is anything but static. In the 1970s when Roe v. Wade was decided, for example, a fetus was considered legally viable starting at 28 weeks. Now, with improved technology and medical management, "the hard limit today is probably 20 or 21 weeks," says Matthew Kemp, associate professor at the University of Western Australia and one of the Australian-Japanese artificial womb project's senior researchers.
The changing threshold can result in situations where lots of people invested in the decision disagree. "Those can be hard decisions, but they are case-by-case decisions that families make or parents make with the key providers to determine when to proceed and when to let the infant die. Usually, it's a shared decision where the parents have the final say," says Johnston. But this isn't always the case.
On May 9th 2016, a boy named Alfie Evans was born in Liverpool, UK. Suffering seizures a few months after his birth, Alfie was diagnosed with an unknown neurodegenerative disorder and soon went into a semi-vegetative state, which lasted for more than a year. Alfie's medical team decided to withdraw his ventilation support, suggesting further treatment was unlawful and inhumane, but his parents wanted permission to fly him to a hospital in Rome and attempt to prolong his life there. In the end, the case went all the way up to the Supreme Court, which ruled that doctors could stop providing life support for Alfie, saying that the child required "peace, quiet and privacy." What happened to little Alfie raised huge publicity in the UK and pointedly highlighted the dilemma of whether parents or doctors should have the final say in the fate of a terminally-ill child in life-support treatment.
"In a few years from now, women who cannot get pregnant because of uterine infertility will be able to have a fully functional uterus made from their own tissue."
Alfie was born and, thus had legal rights, yet legal and ethical mayhem arose out of his case. When it comes to gestatelings, the scenarios will be even more complicated, says Romanis. "I think there's a really big question about who has parental rights and who doesn't," she says. "The assisted reproductive technology (ART) law in the U.K. hasn't been updated since 2008....It certainly needs an update when you think about all the things we have done since [then]."
This June, for instance, scientists from the Wake Forest Institute for Regenerative Medicine in North Carolina published research showing that they could take a small sample of tissue from a rabbit's uterus and create a bioengineered uterus, which then supported both fertilization and normal pregnancy like a natural uterus does.
"In [a number of] years from now, women who cannot get pregnant because of uterine infertility will be able to have a fully functional uterus made from their own tissue," says Dr. Anthony Atala, the Institute's director and a pioneer in regenerative medicine. These bioengineered uteri will eventually be covered by insurance, Atala expects. But when it comes to artificial wombs that externally gestate premature infants, will all mothers have equal access?
Medical reports have already shown racial and ethnic disparities in infertility treatments and access to assisted reproductive technologies. Costs on average total $12,400 per cycle of treatment and may require several cycles to achieve a live birth. "There's no indication that artificial wombs would be treated any differently. That's what we see with almost every expensive new medical technology," says Johnston. In a much more dystopian future, there is even a possibility that inequity in healthcare might create disturbing chasms in how women of various class levels bear children. Romanis asks us to picture the following scenario:
We live in a world where artificial wombs have become mainstream. Most women choose to end their pregnancies early and transfer their gestatelings to the care of machines. After a while, insurers deem full-term pregnancy and childbirth a risky non-necessity, and are lobbying to stop covering them altogether. Wealthy white women continue opting out of their third trimesters (at a high cost), since natural pregnancy has become a substandard route for poorer women. Those women are strongly judged for any behaviors that could risk their fetus's health, in contrast with the machine's controlled environment. "Why are you having a coffee during your pregnancy?" critics might ask. "Why are you having a glass of red wine? If you can't be perfect, why don't you have it the artificial way?"
Problem is, even if they want to, they won't be able to afford it.
In a more sanguine version, however, the artificial wombs are only used in cases of prematurity as a life-saving medical intervention rather than as a lifestyle accommodation. The 15 million babies who are born prematurely each year and may face serious respiratory, cardiovascular, visual and hearing problems, as well as learning disabilities, instead continue their normal development in artificial wombs. After lots of deliberation, insurers agree to bear the cost of external wombs because they are cheaper than a lifetime of medical care for a disabled or diseased person. This enables racial and ethnic minority women, who make up the majority of women giving premature birth, to access the technology.
Even extremely premature babies, those babies (far) below the threshold of 28 weeks of gestation, half of which die, could now discover this thing called life. In this scenario, as the Australian researcher Kemp says, we are simply giving a good shot at healthy, long-term survival to those who were unfortunate enough to start too soon.
Questions remain about new drug for hot flashes
In May, a new drug, Fezolinetant, was approved by the FDA to treat hot flashes associated with menopause.
Vascomotor symptoms (VMS) is the medical term for hot flashes associated with menopause. You are going to hear a lot more about it because a company has a new drug to sell. Here is what you need to know.
Menopause marks the end of a woman’s reproductive capacity. Normal hormonal production associated with that monthly cycle becomes erratic and finally ceases. For some women the transition can be relatively brief with only modest symptoms, while for others the body's “thermostat” in the brain is disrupted and they experience hot flashes and other symptoms that can disrupt daily activity. Lifestyle modification and drugs such as hormone therapy can provide some relief, but women at risk for cancer are advised not to use them and other women choose not to do so.
Fezolinetant, sold by Astellas Pharma Inc. under the product name Veozah™, was approved by the Food and Drug Administration (FDA) on May 12 to treat hot flashes associated with menopause. It is the first in a new class of drugs called neurokinin 3 receptor antagonists, which block specific neurons in the brain “thermostat” that trigger VMS. It does not appear to affect other symptoms of menopause. As with many drugs targeting a brain cell receptor, it must be taken continuously for a few days to build up a good therapeutic response, rather than working as a rescue product such as an asthma inhaler to immediately treat that condition.
Hot flashes vary greatly and naturally get better or resolve completely with time. That contributes to a placebo effect and makes it more difficult to judge the outcome of any intervention. Early this year, a meta analysis of 17 studies of drug trials for hot flashes found an unusually large placebo response in those types of studies; the placebo groups had an average of 5.44 fewer hot flashes and a 36 percent reduction in their severity.
In studies of fezolinetant, the drug recently approved by the FDA, the placebo benefit was strong and persistent. The drug group bested the placebo response to a statistically significant degree but, “If people have gone from 11 hot flashes a day to eight or seven in the placebo group and down to a couple fewer ones in the drug groups, how meaningful is that? Having six hot flashes a day is still pretty unpleasant,” says Diana Zuckerman, president of the National Center for Health Research (NCHR), a health oriented think tank.
“Is a reduction compared to placebo of 2-3 hot flashes per day, in a population of women experiencing 10-11 moderate to severe hot flashes daily, enough relief to be clinically meaningful?” Andrea LaCroix asked a commentary published in Nature Medicine. She is an epidemiologist at the University of California San Diego and a leader of the MsFlash network that has conducted a handful of NIH-funded studies on menopause.
Questions Remain
LaCroix and others have raised questions about how Astellas, the company that makes the new drug, handled missing data from patients who dropped out of the clinical trials. “The lack of detailed information about important parameters such as adherence and missing data raises concerns that the reported benefits of fezolinetant very likely overestimate those that will be observed in clinical practice," LaCroix wrote.
In response to this concern, Anna Criddle, director of global portfolio communications at Astellas, wrote in an email to Leaps.org: “…a full analysis of data, including adherence data and any impact of missing data, was submitted for assessment by [the FDA].”
The company ran the studies at more than 300 sites around the world. Curiously, none appear to have been at academic medical centers, which are known for higher quality research. Zuckerman says, "When somebody is paid to do a study, if they want to get paid to do another study by the same company, they will try to make sure that the results are the results that the company wants.”
Criddle said that Astellas picked the sites “that would allow us to reach a diverse population of women, including race and ethnicity.”
A trial of a lower dose of the drug was conducted in Asia. In March 2022, Astellas issued a press release saying it had failed to prove effectiveness. No further data has been released. Astellas still plans to submit the data, according to Criddle. Results from clinical trials funded by the U.S. goverment must be reported on clinicaltrials.gov within one year of the study's completion - a deadline that, in this case, has expired.
The measurement scale for hot flashes used in the studies, mild-moderate-severe, also came in for criticism. “It is really not good scale, there probably isn’t a broad enough range of things going on or descriptors,” says David Rind. He is chief medical officer of the Institute for Clinical and Economic Review (ICER), a nonprofit authority on new drugs. It conducted a thorough review and analysis of fezolinestant using then existing data gathered from conference abstracts, posters and presentations and included a public stakeholder meeting in December. A 252-page report was published in January, finding “considerable uncertainty about the comparative net health benefits of fezolinetant” versus hormone therapy.
Questions surrounding some of these issues might have been answered if the FDA had chosen to hold a public advisory committee meeting on fezolinetant, which it regularly does for first in class medicines. But the agency decided such a meeting was unnecessary.
Cost
There was little surprise when Astellas announced a list price for fezolinetant of $550 a month ($6000 annually) and a program of patient assistance to ease out of pocket expenses. The company had already incurred large expenses.
In 2017 Astellas purchased the company that originally developed fezolinetant for $534 million plus several hundred million in potential royalties. The drug company ran a "disease awareness” ad, Heat on the Street, hat aired during the Super Bowl in February, where 30 second ads cost about $7 million. Industry analysts have projected sales to be $1.9 billion by 2028.
ICER’s pre-approval evaluation said fezolinetant might "be considered cost-effective if priced around $2,000 annually. ... [It]will depend upon its price and whether it is considered an alternative to MHT [menopause hormone treatment] for all women or whether it will primarily be used by women who cannot or will not take MHT."
Criddle wrote that Astellas set the price based on the novelty of the science, the quality of evidence for the drug and its uniqueness compared to the rest of the market. She noted that an individual’s payment will depend on how much their insurance company decides to cover. “[W]e expect insurance coverage to increase over the course of the year and to achieve widespread coverage in the U.S. over time.”
Leaps.org wrote to and followed up with nine of the largest health insurers/providers asking basic questions about their coverage of fezolinetant. Only two responded. Jennifer Martin, the deputy chief consultant for pharmacy benefits management at the Department of Veterans Affairs, said the agency “covers all drugs from the date that they are launched.” Decisions on whether it will be included in the drug formulary and what if any copays might be required are under review.
“[Fezolinetant] will go through our standard P&T Committee [patient and treatment] review process in the next few months, including a review of available efficacy data, safety data, clinical practice guidelines, and comparison with other agents used for vasomotor symptoms of menopause," said Phil Blando, executive director of corporate communications for CVS Health.
Other insurers likely are going through a similar process to decide issues such as limiting coverage to women who are advised not to use hormones, how much copay will be required, and whether women will be required to first try other options or obtain approvals before getting a prescription.
Rind wants to see a few years of use before he prescribes fezolinetant broadly, and believes most doctors share his view. Nor will they be eager to fill out the additional paperwork required for women to participate in the Astellas patient assistance program, he added.
Safety
Astellas is marketing its drug by pointing out risks of hormone therapy, such as a recent paper in The BMJ, which noted that women who took hormones for even a short period of time had a 24 percent increased risk of dementia. While the percentage was scary, the combined number of women both on and off hormones who developed dementia was small. And it is unclear whether hormones are causing dementia or if more severe hot flashes are a marker for higher risk of developing dementia. This information is emerging only after 80 years of hundreds of millions of women using hormones.
In contrast, the label for fezolinetant prohibits “concomitant use with CYP1A2 inhibitors” and requires testing for liver and kidney function prior to initiating the drug and every three months thereafter. There is no human or animal data on use in a geriatric population, defined as 65 or older, a group that is likely to use the drug. Only a few thousand women have ever taken fezolinetant and most have used it for just a few months.
Options
A woman seeking relief from symptoms of menopause would like to see how fezolintant compares with other available treatment options. But Astellas did not conduct such a study and Andrea LaCroix says it is unlikely that anyone ever will.
ICER has come the closest, with a side-by-side analysis of evidence-based treatments and found that fezolinetant performed quite similarly and modestly as the others in providing relief from hot flashes. Some treatments also help with other symptoms of menopause, which fezolinetant does not.
There are many coping strategies that women can adopt to deal with hot flashes; one of the most common is dressing in layers (such as a sleeveless blouse with a sweater) that can be added or subtracted as conditions require. Avoiding caffeine, hot liquids, and spicy foods is another common strategy. “I stopped drinking hot caffeinated drinks…for several years, and you get out of the habit of drinking them,” says Zuckerman.
LaCroix curates those options at My Meno Plan, which includes a search function where you can enter your symptoms and identify which treatments might work best for you. It also links to published research papers. She says the goal is to empower women with information to make informed decisions about menopause.
A company in England has made a test that picks out the compounds from breath that reveal if people have liver disease.
Every year, around two million people worldwide die of liver disease. While some people inherit the disease, it’s most commonly caused by hepatitis, obesity and alcoholism. These underlying conditions kill liver cells, causing scar tissue to form until eventually the liver cannot function properly. Since 1979, deaths due to liver disease have increased by 400 percent.
The sooner the disease is detected, the more effective treatment can be. But once symptoms appear, the liver is already damaged. Around 50 percent of cases are diagnosed only after the disease has reached the final stages, when treatment is largely ineffective.
To address this problem, Owlstone Medical, a biotech company in England, has developed a breath test that can detect liver disease earlier than conventional approaches. Human breath contains volatile organic compounds (VOCs) that change in the first stages of liver disease. Owlstone’s breath test can reliably collect, store and detect VOCs, while picking out the specific compounds that reveal liver disease.
“There’s a need to screen more broadly for people with early-stage liver disease,” says Owlstone’s CEO Billy Boyle. “Equally important is having a test that's non-invasive, cost effective and can be deployed in a primary care setting.”
The standard tool for detection is a biopsy. It is invasive and expensive, making it impractical to use for people who aren't yet symptomatic. Meanwhile, blood tests are less invasive, but they can be inaccurate and can’t discriminate between different stages of the disease.
In the past, breath tests have not been widely used because of the difficulties of reliably collecting and storing breath. But Owlstone’s technology could help change that.
The team is testing patients in the early stages of advanced liver disease, or cirrhosis, to identify and detect these biomarkers. In an initial study, Owlstone’s breathalyzer was able to pick out patients who had early cirrhosis with 83 percent sensitivity.
Boyle’s work is personally motivated. His wife died of colorectal cancer after she was diagnosed with a progressed form of the disease. “That was a big impetus for me to see if this technology could work in early detection,” he says. “As a company, Owlstone is interested in early detection across a range of diseases because we think that's a way to save lives and a way to save costs.”
How it works
In the past, breath tests have not been widely used because of the difficulties of reliably collecting and storing breath. But Owlstone’s technology could help change that.
Study participants breathe into a mouthpiece attached to a breath sampler developed by Owlstone. It has cartridges are designed and optimized to collect gases. The sampler specifically targets VOCs, extracting them from atmospheric gases in breath, to ensure that even low levels of these compounds are captured.
The sampler can store compounds stably before they are assessed through a method called mass spectrometry, in which compounds are converted into charged atoms, before electromagnetic fields filter and identify even the tiniest amounts of charged atoms according to their weight and charge.
The top four compounds in our breath
In an initial study, Owlstone captured VOCs in breath to see which ones could help them tell the difference between people with and without liver disease. They tested the breath of 46 patients with liver disease - most of them in the earlier stages of cirrhosis - and 42 healthy people. Using this data, they were able to create a diagnostic model. Individually, compounds like 2-Pentanone and limonene performed well as markers for liver disease. Owlstone achieved even better performance by examining the levels of the top four compounds together, distinguishing between liver disease cases and controls with 95 percent accuracy.
“It was a good proof of principle since it looks like there are breath biomarkers that can discriminate between diseases,” Boyle says. “That was a bit of a stepping stone for us to say, taking those identified, let’s try and dose with specific concentrations of probes. It's part of building the evidence and steering the clinical trials to get to liver disease sensitivity.”
Sabine Szunerits, a professor of chemistry in Institute of Electronics at the University of Lille, sees the potential of Owlstone’s technology.
“Breath analysis is showing real promise as a clinical diagnostic tool,” says Szunerits, who has no ties with the company. “Owlstone Medical’s technology is extremely effective in collecting small volatile organic biomarkers in the breath. In combination with pattern recognition it can give an answer on liver disease severity. I see it as a very promising way to give patients novel chances to be cured.”
Improving the breath sampling process
Challenges remain. With more than one thousand VOCs found in the breath, it can be difficult to identify markers for liver disease that are consistent across many patients.
Julian Gardner is a professor of electrical engineering at Warwick University who researches electronic sensing devices. “Everyone’s breath has different levels of VOCs and different ones according to gender, diet, age etc,” Gardner says. “It is indeed very challenging to selectively detect the biomarkers in the breath for liver disease.”
So Owlstone is putting chemicals in the body that they know interact differently with patients with liver disease, and then using the breath sampler to measure these specific VOCs. The chemicals they administer are called Exogenous Volatile Organic Compound) probes, or EVOCs.
Most recently, they used limonene as an EVOC probe, testing 29 patients with early cirrhosis and 29 controls. They gave the limonene to subjects at specific doses to measure how its concentrations change in breath. The aim was to try and see what was happening in their livers.
“They are proposing to use drugs to enhance the signal as they are concerned about the sensitivity and selectivity of their method,” Gardner says. “The approach of EVOC probes is probably necessary as you can then eliminate the person-to-person variation that will be considerable in the soup of VOCs in our breath.”
Through these probes, Owlstone could identify patients with liver disease with 83 percent sensitivity. By targeting what they knew was a disease mechanism, they were able to amplify the signal. The company is starting a larger clinical trial, and the plan is to eventually use a panel of EVOC probes to make sure they can see diverging VOCs more clearly.
“I think the approach of using probes to amplify the VOC signal will ultimately increase the specificity of any VOC breath tests, and improve their practical usability,” says Roger Yazbek, who leads the South Australian Breath Analysis Research (SABAR) laboratory in Flinders University. “Whilst the findings are interesting, it still is only a small cohort of patients in one location.”
The future of breath diagnosis
Owlstone wants to partner with pharmaceutical companies looking to learn if their drugs have an effect on liver disease. They’ve also developed a microchip, a miniaturized version of mass spectrometry instruments, that can be used with the breathalyzer. It is less sensitive but will enable faster detection.
Boyle says the company's mission is for their tests to save 100,000 lives. "There are lots of risks and lots of challenges. I think there's an opportunity to really establish breath as a new diagnostic class.”

