Scientists Are Devising Clever Solutions to Feed Astronauts on Mars Space Flights

Astronaut and Expedition 64 Flight Engineer Soichi Noguchi of the Japan Aerospace Exploration Agency displays Extra Dwarf Pak Choi plants growing aboard the International Space Station. The plants were grown for the Veggie study which is exploring space agriculture as a way to sustain astronauts on future missions to the Moon or Mars.
Astronauts at the International Space Station today depend on pre-packaged, freeze-dried food, plus some fresh produce thanks to regular resupply missions. This supply chain, however, will not be available on trips further out, such as the moon or Mars. So what are astronauts on long missions going to eat?
Going by the options available now, says Christel Paille, an engineer at the European Space Agency, a lunar expedition is likely to have only dehydrated foods. “So no more fresh product, and a limited amount of already hydrated product in cans.”
For the Mars mission, the situation is a bit more complex, she says. Prepackaged food could still constitute most of their food, “but combined with [on site] production of certain food products…to get them fresh.” A Mars mission isn’t right around the corner, but scientists are currently working on solutions for how to feed those astronauts. A number of boundary-pushing efforts are now underway.
The logistics of growing plants in space, of course, are very different from Earth. There is no gravity, sunlight, or atmosphere. High levels of ionizing radiation stunt plant growth. Plus, plants take up a lot of space, something that is, ironically, at a premium up there. These and special nutritional requirements of spacefarers have given scientists some specific and challenging problems.
To study fresh food production systems, NASA runs the Vegetable Production System (Veggie) on the ISS. Deployed in 2014, Veggie has been growing salad-type plants on “plant pillows” filled with growth media, including a special clay and controlled-release fertilizer, and a passive wicking watering system. They have had some success growing leafy greens and even flowers.
"Ideally, we would like a system which has zero waste and, therefore, needs zero input, zero additional resources."
A larger farming facility run by NASA on the ISS is the Advanced Plant Habitat to study how plants grow in space. This fully-automated, closed-loop system has an environmentally controlled growth chamber and is equipped with sensors that relay real-time information about temperature, oxygen content, and moisture levels back to the ground team at Kennedy Space Center in Florida. In December 2020, the ISS crew feasted on radishes grown in the APH.
“But salad doesn’t give you any calories,” says Erik Seedhouse, a researcher at the Applied Aviation Sciences Department at Embry-Riddle Aeronautical University in Florida. “It gives you some minerals, but it doesn’t give you a lot of carbohydrates.” Seedhouse also noted in his 2020 book Life Support Systems for Humans in Space: “Integrating the growing of plants into a life support system is a fiendishly difficult enterprise.” As a case point, he referred to the ESA’s Micro-Ecological Life Support System Alternative (MELiSSA) program that has been running since 1989 to integrate growing of plants in a closed life support system such as a spacecraft.
Paille, one of the scientists running MELiSSA, says that the system aims to recycle the metabolic waste produced by crew members back into the metabolic resources required by them: “The aim is…to come [up with] a closed, sustainable system which does not [need] any logistics resupply.” MELiSSA uses microorganisms to process human excretions in order to harvest carbon dioxide and nitrate to grow plants. “Ideally, we would like a system which has zero waste and, therefore, needs zero input, zero additional resources,” Paille adds.
Microorganisms play a big role as “fuel” in food production in extreme places, including in space. Last year, researchers discovered Methylobacterium strains on the ISS, including some never-seen-before species. Kasthuri Venkateswaran of NASA’s Jet Propulsion Laboratory, one of the researchers involved in the study, says, “[The] isolation of novel microbes that help to promote the plant growth under stressful conditions is very essential… Certain bacteria can decompose complex matter into a simple nutrient [that] the plants can absorb.” These microbes, which have already adapted to space conditions—such as the absence of gravity and increased radiation—boost various plant growth processes and help withstand the harsh physical environment.
MELiSSA, says Paille, has demonstrated that it is possible to grow plants in space. “This is important information because…we didn’t know whether the space environment was affecting the biological cycle of the plant…[and of] cyanobacteria.” With the scientific and engineering aspects of a closed, self-sustaining life support system becoming clearer, she says, the next stage is to find out if it works in space. They plan to run tests recycling human urine into useful components, including those that promote plant growth.
The MELiSSA pilot plant uses rats currently, and needs to be translated for human subjects for further studies. “Demonstrating the process and well-being of a rat in terms of providing water, sufficient oxygen, and recycling sufficient carbon dioxide, in a non-stressful manner, is one thing,” Paille says, “but then, having a human in the loop [means] you also need to integrate user interfaces from the operational point of view.”
Growing food in space comes with an additional caveat that underscores its high stakes. Barbara Demmig-Adams from the Department of Ecology and Evolutionary Biology at the University of Colorado Boulder explains, “There are conditions that actually will hurt your health more than just living here on earth. And so the need for nutritious food and micronutrients is even greater for an astronaut than for [you and] me.”
Demmig-Adams, who has worked on increasing the nutritional quality of plants for long-duration spaceflight missions, also adds that there is no need to reinvent the wheel. Her work has focused on duckweed, a rather unappealingly named aquatic plant. “It is 100 percent edible, grows very fast, it’s very small, and like some other floating aquatic plants, also produces a lot of protein,” she says. “And here on Earth, studies have shown that the amount of protein you get from the same area of these floating aquatic plants is 20 times higher compared to soybeans.”
Aquatic plants also tend to grow well in microgravity: “Plants that float on water, they don’t respond to gravity, they just hug the water film… They don’t need to know what’s up and what’s down.” On top of that, she adds, “They also produce higher concentrations of really important micronutrients, antioxidants that humans need, especially under space radiation.” In fact, duckweed, when subjected to high amounts of radiation, makes nutrients called carotenoids that are crucial for fighting radiation damage. “We’ve looked at dozens and dozens of plants, and the duckweed makes more of this radiation fighter…than anything I’ve seen before.”
Despite all the scientific advances and promising leads, no one really knows what the conditions so far out in space will be and what new challenges they will bring. As Paille says, “There are known unknowns and unknown unknowns.”
One definite “known” for astronauts is that growing their food is the ideal scenario for space travel in the long term since “[taking] all your food along with you, for best part of two years, that’s a lot of space and a lot of weight,” as Seedhouse says. That said, once they land on Mars, they’d have to think about what to eat all over again. “Then you probably want to start building a greenhouse and growing food there [as well],” he adds.
And that is a whole different challenge altogether.
A model of a human kidney.
Science's dream of creating perfect custom organs on demand as soon as a patient needs one is still a long way off. But tiny versions are already serving as useful research tools and stepping stones toward full-fledged replacements.
Although organoids cannot yet replace kidneys, they are invaluable tools for research.
The Lowdown
Australian researchers have grown hundreds of mini human kidneys in the past few years. Known as organoids, they function much like their full-grown counterparts, minus a few features due to a lack of blood supply.
Cultivated in a petri dish, these kidneys are still a shadow of their human counterparts. They grow no larger than one-sixth of an inch in diameter; fully developed organs are up to five inches in length. They contain no more than a few dozen nephrons, the kidney's individual blood-filtering unit, whereas a fully-grown kidney has about 1 million nephrons. And the dish variety live for just a few weeks.
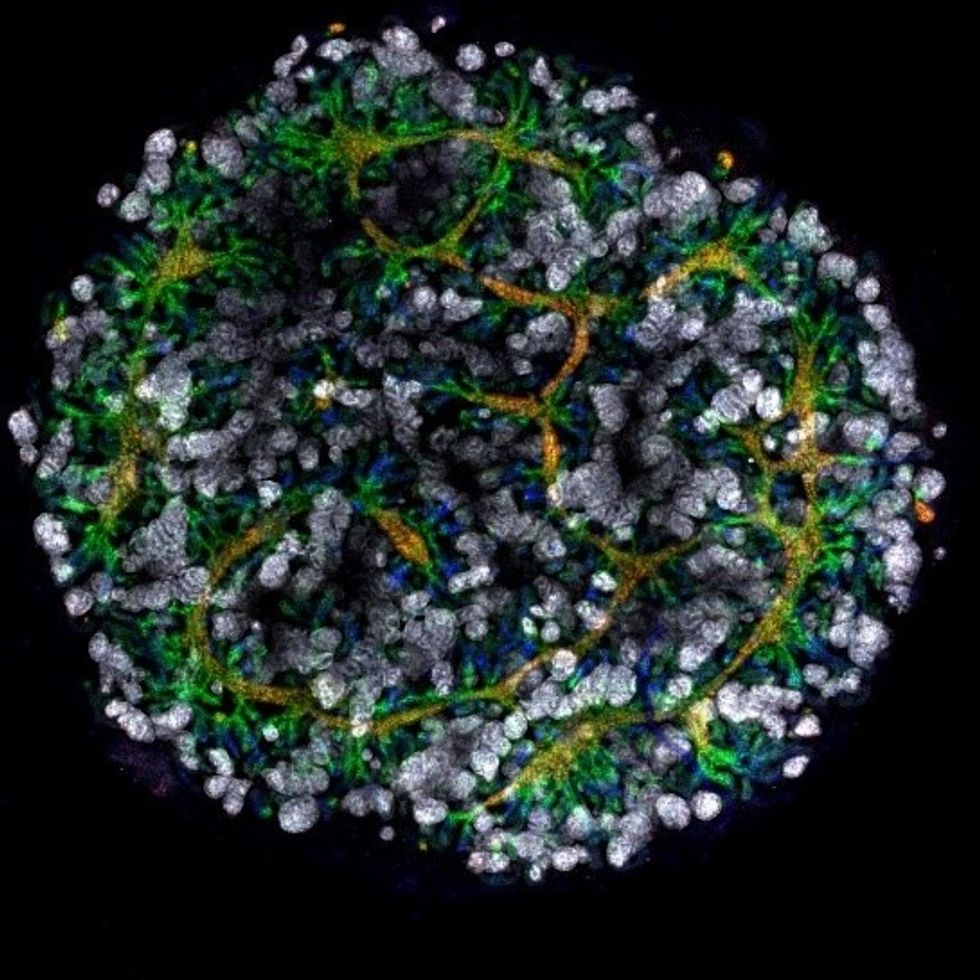
An organoid kidney created by the Murdoch Children's Institute in Melbourne, Australia.
Photo Credit: Shahnaz Khan.
But Melissa Little, head of the kidney research laboratory at the Murdoch Children's Institute in Melbourne, says these organoids are invaluable tools for research. Although renal failure is rare in children, more than half of those who suffer from such a disorder inherited it.
The mini kidneys enable scientists to better understand the progression of such disorders because they can be grown with a patient's specific genetic condition.
Mature stem cells can be extracted from a patient's blood sample and then reprogrammed to become like embryonic cells, able to turn into any type of cell in the body. It's akin to walking back the clock so that the cells regain unlimited potential for development. (The Japanese scientist who pioneered this technique was awarded the Nobel Prize in 2012.) These "induced pluripotent stem cells" can then be chemically coaxed to grow into mini kidneys that have the patient's genetic disorder.
"The (genetic) defects are quite clear in the organoids, and they can be monitored in the dish," Little says. To date, her research team has created organoids from 20 different stem cell lines.
Medication regimens can also be tested on the organoids, allowing specific tailoring for each patient. For now, such testing remains restricted to mice, but Little says it eventually will be done on human organoids so that the results can more accurately reflect how a given patient will respond to particular drugs.
Next Steps
Although these organoids cannot yet replace kidneys, Little says they may plug a huge gap in renal care by assisting in developing new treatments for chronic conditions. Currently, most patients with a serious kidney disorder see their options narrow to dialysis or organ transplantation. The former not only requires multiple sessions a week, but takes a huge toll on patient health.
Ten percent of older patients on dialysis die every year in the U.S. Aside from the physical trauma of organ transplantation, finding a suitable donor outside of a family member can be difficult.
"This is just another great example of the potential of pluripotent stem cells."
Meanwhile, the ongoing creation of organoids is supplying Little and her colleagues with enough information to create larger and more functional organs in the future. According to Little, researchers in the Netherlands, for example, have found that implanting organoids in mice leads to the creation of vascular growth, a potential pathway toward creating bigger and better kidneys.
And while Little acknowledges that creating a fully-formed custom organ is the ultimate goal, the mini organs are an important bridge step.
"This is just another great example of the potential of pluripotent stem cells, and I am just passionate to see it do some good."
Phil Gutis, an Alzheimer's patient who participated in a failed clinical trial, poses with his dog Abe.
Phil Gutis never had a stellar memory, but when he reached his early 50s, it became a problem he could no longer ignore. He had trouble calculating how much to tip after a meal, finding things he had just put on his desk, and understanding simple driving directions.
From 1998-2017, industry sources reported 146 failed attempts at developing Alzheimer's drugs.
So three years ago, at age 54, he answered an ad for a drug trial seeking people experiencing memory issues. He scored so low in the memory testing he was told something was wrong. M.R.I.s and PET scans confirmed that he had early-onset Alzheimer's disease.
Gutis, who is a former New York Times reporter and American Civil Liberties Union spokesman, felt fortunate to get into an advanced clinical trial of a new treatment for Alzheimer's disease. The drug, called aducanumab, had shown promising results in earlier studies.
Four years of data had found that the drug effectively reduced the burden of protein fragments called beta-amyloids, which destroy connections between nerve cells. Amyloid plaques are found in the brains of patients with Alzheimer's disease and are associated with impairments in thinking and memory.
Gutis eagerly participated in the clinical trial and received 35 monthly infusions. "For the first 20 infusions, I did not know whether I was receiving the drug or the placebo," he says. "During the last 15 months, I received aducanumab. But it really didn't matter if I was receiving the drug or the placebo because on March 21, the trial was stopped because [the drug company] Biogen found that the treatments were ineffective."
The news was devastating to the trial participants, but also to the Alzheimer's research community. Earlier this year, another pharmaceutical company, Roche, announced it was discontinuing two of its Alzheimer's clinical trials. From 1998-2017, industry sources reported 146 failed attempts at developing Alzheimer's drugs. There are five prescription drugs approved to treat its symptoms, but a cure remains elusive. The latest failures have left researchers scratching their heads about how to approach attacking the disease.
The failure of aducanumab was also another setback for the estimated 5.8 million people who have Alzheimer's in the United States. Of these, around 5.6 million are older than 65 and 200,000 suffer from the younger-onset form, including Gutis.
Gutis is understandably distraught about the cancellation of the trial. "I really had hopes it would work. So did all the patients."
While drug companies have failed so far, another group is stepping up to expedite the development of a cure: venture philanthropists.
For now, he is exercising every day to keep his blood flowing, which is supposed to delay the progression of the disease, and trying to eat a low-fat diet. "But I know that none of it will make a difference. Alzheimer's is a progressive disease. There are no treatments to delay it, let alone cure it."
But while drug companies have failed so far, another group is stepping up to expedite the development of a cure: venture philanthropists. These are successful titans of industry and dedicated foundations who are donating large sums of money to fill a much-needed void – funding research to look for new biomarkers.
Biomarkers are neurochemical indicators that can be used to detect the presence of a disease and objectively measure its progression. There are currently no validated biomarkers for Alzheimer's, but researchers are actively studying promising candidates. The hope is that they will find a reliable way to identify the disease even before the symptoms of mental decline show up, so that treatments can be directed at a very early stage.
Howard Fillit, Founding Executive Director and Chief Science Officer of the Alzheimer's Drug Discovery Foundation, says, "We need novel biomarkers to diagnose Alzheimer's disease and related dementias. But pharmaceutical companies don't put money into biomarkers research."
One of the venture philanthropists who has recently stepped up to the task is Bill Gates. In January 2018, he announced his father had Alzheimer's disease in an interview on the Today Show with Maria Shriver, whose father Sargent Shriver, died of Alzheimer's disease in 2011. Gates told Ms. Shriver that he had invested $100 million into Alzheimer's research, with $50 million of his donation going to Dementia Discovery Fund, which looks for new cures and treatments.
That August, Gates joined other investors in a new fund called Diagnostics Accelerator. The project aims to supports researchers looking to speed up new ideas for earlier and better diagnosis of the disease.
Gates and other donors committed more than $35 million to help launch it, and this April, Jeff and Mackenzie Bezos joined the coalition, bringing the current program funding to nearly $50 million.
"It makes sense that a challenge this significant would draw the attention of some of the world's leading thinkers."
None of these funders stand to make a profit on their donation, unlike traditional research investments by drug companies. The standard alternatives to such funding have upsides -- and downsides.
As Bill Gates wrote on his blog, "Investments from governments or charitable organizations are fantastic at generating new ideas and cutting-edge research -- but they're not always great at creating usable products, since no one stands to make a profit at the end of the day.
"Venture capital, on the other end of the spectrum, is more likely to develop a test that will reach patients, but its financial model favors projects that will earn big returns for investors. Venture philanthropy splits the difference. It incentivizes a bold, risk-taking approach to research with an end goal of a real product for real patients. If any of the projects backed by Diagnostics Accelerator succeed, our share of the financial windfall goes right back into the fund."
Gutis said he is thankful for any attention given to finding a cure for Alzheimer's.
"Most doctors and scientists will tell you that we're still in the dark ages when it comes to fully understanding how the brain works, let alone figuring out the cause or treatment for Alzheimer's.
"It makes sense that a challenge this significant would draw the attention of some of the world's leading thinkers. I only hope they can be more successful with their entrepreneurial approach to finding a cure than the drug companies have been with their more traditional paths."