At the “Apple Store of Doctor’s Offices,” Preventive Care Is High Tech. Is it Worth $150 a Month?
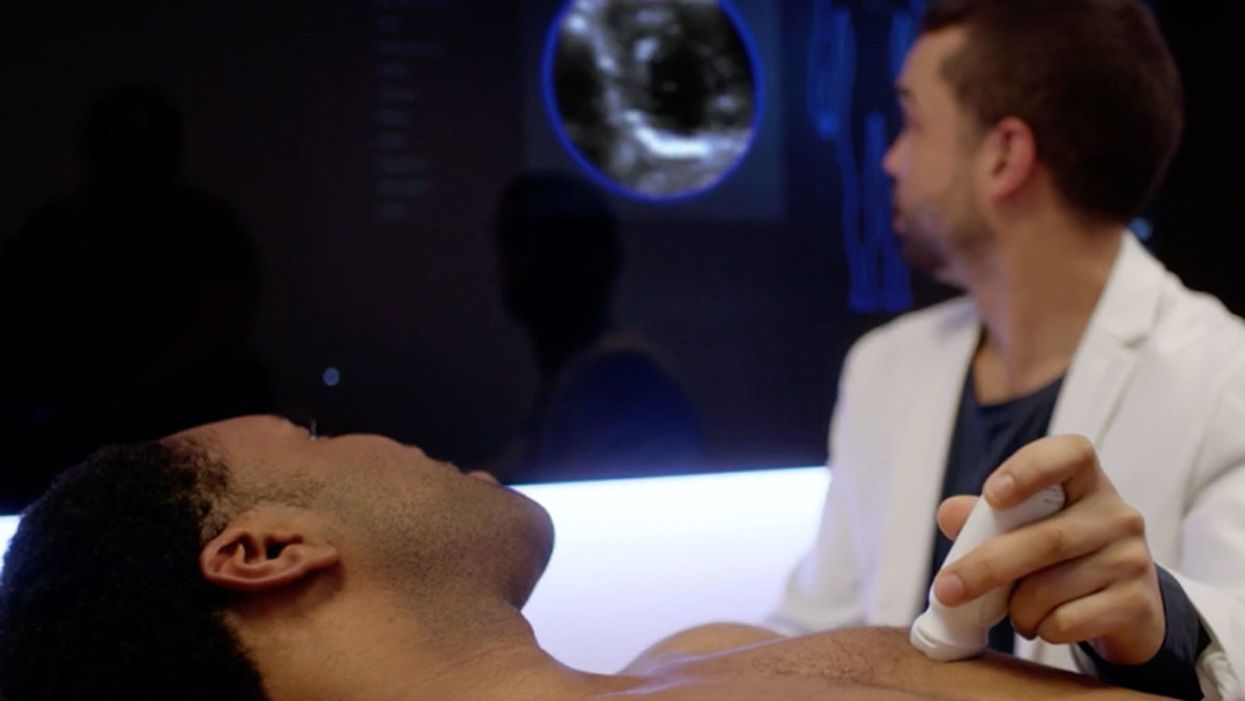
A patient getting examined at health startup Forward.
What if going to the doctor's office could be … nice?
If you didn't have to wait for your appointment, but were ushered right in; if your medical data was all collated and easily searchable on an iPhone app; if a remote scribe took notes while you spoke with your doctor so you could make eye contact with them; if your doctor didn't seem horribly rushed.
Would you go to the doctor to get help staying healthy, rather than just to stop being sick?
Would that change the way you thought about your health? Would you go to the doctor to get help staying healthy, rather than just to stop being sick? And would that, in the long run, be much better for you?
Those are the animating questions for Forward, a healthcare startup devoted to preventive care. Led by founder Adrian Aoun, formerly of Google/Sidewalk labs, Forward opened its first office in San Francisco in 2016 and has since expanded to Los Angeles, Orange County, New York, and Washington, D.C., with a San Diego location opening soon.
It's been described as the "Apple Store of doctor's offices," which in some ways is a reaction to Forward's vibe: Patients have described the offices as having blonde wood, minimalist design, sparkling water on tap — and lots of high-tech gadgets, like the full-body scanner that replaces the standard scale and stethoscope.

The interior of a Forward office.
(Courtesy Forward)
The more crucial difference, though, is its model of care. Forward doesn't take insurance. Instead, patients, or "members," pay a flat $149 per month, along the lines of a subscription service like Netflix or a gym membership. That fee covers visits, messaging with medical staff through the Forward app, the use of a wearable (like a Fitbit or a sleep tracker) if the physician recommends it, plus any bloodwork or diagnostic tests run in the on-site labs. (The company declined to disclose how many people have signed up for memberships.)
Predictability is Forward's other significant, distinguishing feature: No surprise co-pays, or extra charges showing up on a billing statement months later. Everything is wrapped up in the $149 membership fee, unless the physician recommends visiting an outside specialist.
That caveat isn't a small one. It's important to note that Forward is in no way meant to replace standard health insurance. The service is strictly focused on preventive care, so it wouldn't be much use in case of an emergency; it's meant to help people, as far as is possible, avoid that emergency at all.
Ani Okkasian's family recently went through such an emergency. Her 62-year-old father, an active and seemingly healthy man living with diabetes, had been feeling unwell for a while, but struggled to receive constructive follow-up or tests from his doctor. It finally emerged that his liver was severely damaged, and he suffered a stroke — the risk of which can be elevated by liver disease. He seemed to deteriorate completely within mere weeks, Okkasian said, and in January he passed away.
"He was someone who'd go to the doctor regularly and listen to what they said and follow it," Okkasian said. "I shouldn't have had to bury my father at 62. I still believe to my core that his death could have been avoided if the primary care was adequate."
"I could tell that the people who designed [Forward] had lost someone to the legacy system; it was so streamlined and so much clearer."
Okkasian began researching, looking for a better alternative, and discovered Forward. Founder Aoun lost his grandfather to a heart attack; his brother's heart attack at age 31 was the impetus to start Forward.
"I could tell that that was the genesis," Okkasian said. "Having just lost someone, and having had to deal with different aspects of the healthcare industry — how complicated and convoluted that all is — I could tell that the people who designed [Forward] had lost someone to the legacy system; it was so streamlined and so much clearer."
So Who Is Forward For?
The Affordable Care Act mandates that evidence-based preventive care must be covered by insurers without any cost to the patient. Today, 30 million Americans are still living without health insurance; but for most of the population, cost shouldn't prevent access to standard, preventive care, says Benjamin Sommers, a physician and professor at the Harvard T.H. Chan School of Public Health who has studied the effect of the ACA on preventive care access.
For Okkasian and her family, it wasn't a lack of access to primary care that was at issue; it was the quality of that primary care. In 2019, that's probably true for a lot of people.
"How come all other industries have been disturbed except the medical industry?" Okkasian asked. "It's disturbing the most people. We're so advanced in so many ways, but when it comes to the healthcare system, we're not prioritizing the wellness of a person."
Is Forward the answer? Well, probably not for everyone. Its office are only in a handful of cities, and there are limits to how scalable it would be; it's unavoidable that the $149 per month charge restricts access for a lot of people. Those who have insurance through their employer might have a flexible spending account (FSA) that would cover some or all of the membership fee, and Forward has said that 15 percent of their early members came from underserved communities and were offered free plans; but for many others, that's just an unworkable extra cost.
Sommers also sounded a dubious note about a maximalist attitude toward data collection.
"Even though some patients may think that 'more is always better' — more testing, more screening, etc. — this isn't true," he said. "Some types of cancer screening, ovarian cancer screening for instance, are actually harmful or of no benefit, because studies have shown that they don't improve survival or health outcomes, but can lead to unnecessary testing, pain, false positives, anxiety, and other side effects.
"It's really great for people who are in good health, looking to make it better."
"I'm generally skeptical of efforts to charge people more to get 'extra testing' that isn't currently supported by the medical evidence," he added.
But relatively healthy people who want to take a more active approach to their health — or people who have frequent testing needs, like those using the HIV-prevention drug PrEP, and want to avoid co-pays — might benefit from the on-demand, low-friction experience that Forward offers.
"It's really great for people who are in good health, looking to make it better," Okkasian said. "Your experience is simplified to a point where you feel empowered, not scared."
A new type of cancer therapy is shrinking deadly brain tumors with just one treatment
MRI scans after a new kind of immunotherapy for brain cancer show remarkable progress in one patient just days after the first treatment.
Few cancers are deadlier than glioblastomas—aggressive and lethal tumors that originate in the brain or spinal cord. Five years after diagnosis, less than five percent of glioblastoma patients are still alive—and more often, glioblastoma patients live just 14 months on average after receiving a diagnosis.
But an ongoing clinical trial at Mass General Cancer Center is giving new hope to glioblastoma patients and their families. The trial, called INCIPIENT, is meant to evaluate the effects of a special type of immune cell, called CAR-T cells, on patients with recurrent glioblastoma.
How CAR-T cell therapy works
CAR-T cell therapy is a type of cancer treatment called immunotherapy, where doctors modify a patient’s own immune system specifically to find and destroy cancer cells. In CAR-T cell therapy, doctors extract the patient’s T-cells, which are immune system cells that help fight off disease—particularly cancer. These T-cells are harvested from the patient and then genetically modified in a lab to produce proteins on their surface called chimeric antigen receptors (thus becoming CAR-T cells), which makes them able to bind to a specific protein on the patient’s cancer cells. Once modified, these CAR-T cells are grown in the lab for several weeks so that they can multiply into an army of millions. When enough cells have been grown, these super-charged T-cells are infused back into the patient where they can then seek out cancer cells, bind to them, and destroy them. CAR-T cell therapies have been approved by the US Food and Drug Administration (FDA) to treat certain types of lymphomas and leukemias, as well as multiple myeloma, but haven’t been approved to treat glioblastomas—yet.
CAR-T cell therapies don’t always work against solid tumors, such as glioblastomas. Because solid tumors contain different kinds of cancer cells, some cells can evade the immune system’s detection even after CAR-T cell therapy, according to a press release from Massachusetts General Hospital. For the INCIPIENT trial, researchers modified the CAR-T cells even further in hopes of making them more effective against solid tumors. These second-generation CAR-T cells (called CARv3-TEAM-E T cells) contain special antibodies that attack EFGR, a protein expressed in the majority of glioblastoma tumors. Unlike other CAR-T cell therapies, these particular CAR-T cells were designed to be directly injected into the patient’s brain.
The INCIPIENT trial results
The INCIPIENT trial involved three patients who were enrolled in the study between March and July 2023. All three patients—a 72-year-old man, a 74-year-old man, and a 57-year-old woman—were treated with chemo and radiation and enrolled in the trial with CAR-T cells after their glioblastoma tumors came back.
The results, which were published earlier this year in the New England Journal of Medicine (NEJM), were called “rapid” and “dramatic” by doctors involved in the trial. After just a single infusion of the CAR-T cells, each patient experienced a significant reduction in their tumor sizes. Just two days after receiving the infusion, the glioblastoma tumor of the 72-year-old man decreased by nearly twenty percent. Just two months later the tumor had shrunk by an astonishing 60 percent, and the change was maintained for more than six months. The most dramatic result was in the 57-year-old female patient, whose tumor shrank nearly completely after just one infusion of the CAR-T cells.
The results of the INCIPIENT trial were unexpected and astonishing—but unfortunately, they were also temporary. For all three patients, the tumors eventually began to grow back regardless of the CAR-T cell infusions. According to the press release from MGH, the medical team is now considering treating each patient with multiple infusions or prefacing each treatment with chemotherapy to prolong the response.
While there is still “more to do,” says co-author of the study neuro-oncologist Dr. Elizabeth Gerstner, the results are still promising. If nothing else, these second-generation CAR-T cell infusions may someday be able to give patients more time than traditional treatments would allow.
“These results are exciting but they are also just the beginning,” says Dr. Marcela Maus, a doctor and professor of medicine at Mass General who was involved in the clinical trial. “They tell us that we are on the right track in pursuing a therapy that has the potential to change the outlook for this intractable disease.”
A recent study in The Lancet Oncology showed that AI found 20 percent more cancers on mammogram screens than radiologists alone.
Since the early 2000s, AI systems have eliminated more than 1.7 million jobs, and that number will only increase as AI improves. Some research estimates that by 2025, AI will eliminate more than 85 million jobs.
But for all the talk about job security, AI is also proving to be a powerful tool in healthcare—specifically, cancer detection. One recently published study has shown that, remarkably, artificial intelligence was able to detect 20 percent more cancers in imaging scans than radiologists alone.
Published in The Lancet Oncology, the study analyzed the scans of 80,000 Swedish women with a moderate hereditary risk of breast cancer who had undergone a mammogram between April 2021 and July 2022. Half of these scans were read by AI and then a radiologist to double-check the findings. The second group of scans was read by two researchers without the help of AI. (Currently, the standard of care across Europe is to have two radiologists analyze a scan before diagnosing a patient with breast cancer.)
The study showed that the AI group detected cancer in 6 out of every 1,000 scans, while the radiologists detected cancer in 5 per 1,000 scans. In other words, AI found 20 percent more cancers than the highly-trained radiologists.

But even though the AI was better able to pinpoint cancer on an image, it doesn’t mean radiologists will soon be out of a job. Dr. Laura Heacock, a breast radiologist at NYU, said in an interview with CNN that radiologists do much more than simply screening mammograms, and that even well-trained technology can make errors. “These tools work best when paired with highly-trained radiologists who make the final call on your mammogram. Think of it as a tool like a stethoscope for a cardiologist.”
AI is still an emerging technology, but more and more doctors are using them to detect different cancers. For example, researchers at MIT have developed a program called MIRAI, which looks at patterns in patient mammograms across a series of scans and uses an algorithm to model a patient's risk of developing breast cancer over time. The program was "trained" with more than 200,000 breast imaging scans from Massachusetts General Hospital and has been tested on over 100,000 women in different hospitals across the world. According to MIT, MIRAI "has been shown to be more accurate in predicting the risk for developing breast cancer in the short term (over a 3-year period) compared to traditional tools." It has also been able to detect breast cancer up to five years before a patient receives a diagnosis.
The challenges for cancer-detecting AI tools now is not just accuracy. AI tools are also being challenged to perform consistently well across different ages, races, and breast density profiles, particularly given the increased risks that different women face. For example, Black women are 42 percent more likely than white women to die from breast cancer, despite having nearly the same rates of breast cancer as white women. Recently, an FDA-approved AI device for screening breast cancer has come under fire for wrongly detecting cancer in Black patients significantly more often than white patients.
As AI technology improves, radiologists will be able to accurately scan a more diverse set of patients at a larger volume than ever before, potentially saving more lives than ever.

