The CRISPR-Cas9 gene editing tool could be used to "turn off" pain directly, raising ethical questions for society.
Scientists have long been aware that some people live with what's known as "congenital insensitivity to pain"—the inability to register the tingles, jolts, and aches that alert most people to injury or illness.
"If you break the chain of transmission somewhere along there, it doesn't matter what the message is—the recipient will not get it."
On the ospposite end of the spectrum, others suffer from hyperalgesia, or extreme pain; for those with erythromelalgia, also known as "Man on Fire Syndrome," warm temperatures can feel like searing heat—even wearing socks and shoes can make walking unbearable.
Strangely enough, the two conditions can be traced to mutations in the same gene, SCN9A. It produces a protein that exists in spinal cells—specifically, in the dorsal root ganglion—which transmits the sensation of pain from the nerves at the peripheral site of an injury into the central nervous system and to the brain. This fact may become the key to pain relief for the roughly 20 percent of Americans who suffer from chronic pain, and countless other patients around the world.
"If you break the chain of transmission somewhere along there, it doesn't matter what the message is—the recipient will not get it," said Dr. Fyodor Urnov, director of the Innovative Genomics Institute and a professor of molecular and cell biology at the University of California, Berkeley. "For scientists and clinicians who study this, [there's] this consistent tracking of: You break this gene, you stop feeling pain; make this gene hyperactive, you feel lots of pain—that really cuts through the correlation versus causation question."
Researchers tried for years, without much success, to find a chemical that would block that protein from working and therefore mute the pain sensation. The CRISPR-Cas9 gene editing tool could completely sidestep that approach and "turn off" pain directly.
Yet as CRISPR makes such targeted therapies increasingly possible, the ethical questions surrounding gene editing have taken on a new and more urgent cast—particularly in light of the work of the disgraced Chinese scientist He Jiankui, who announced in late 2018 that he had created the world's first genetically edited babies. He used CRISPR to edit two embryos, with the goal of disabling a gene that makes people susceptible to HIV infection; but then took the unprecedented step of implanting the edited embryos for pregnancy and birth.
Edits to germline cells, like the ones He undertook, involve alterations to gametes or embryos and carry much higher risk than somatic cell edits, since changes will be passed on to any future generations. There are also concerns that imprecise edits could result in mutations and end up causing more disorders. Recent developments, particularly the "search-and replace" prime-editing technique published last fall, will help minimize those accidental edits, but the fact remains that we have little understanding of the long-term effects of these germline edits—for the future of the patients themselves, or for the broader gene pool.
"We need to have appropriate venues where we deliberate and consider the ethical, legal and social implications of gene editing as a society."
It is much harder to predict the effects, harmful or otherwise, on the larger human population as a result of interactions with the environment or other genetic variations; with somatic cell edits, on the other hand— like the ones that would be made in an individual to turn off pain—only the person receiving the treatment is affected.
Beyond the somatic/germline distinction, there is also a larger ethical question over how much genetic interference society is willing to tolerate, which may be couched as the difference between therapeutic editing—interventions in response to a demonstrated medical need—and "enhancement" editing. The Chinese scientist He was roundly criticized in the scientific community for the fact that there are already much safer and more proven methods of preventing the parent-to-child transmission of HIV through the IVF process, making his genetic edits medically unnecessary. (The edits may also have increased the girls' risk of susceptibility to other viruses, like influenza and the West Nile virus.)
Yet there are even more extreme goals that CRISPR could be used to reach, ones further removed from any sort of medical treatment. The 1997 science fiction movie Gattaca imagined a dystopian future where genetic selection for strength and intelligence is common, creating a society that explicitly and unapologetically endorses eugenics. In the real world, Russian President Vladimir Putin has commented that genetic editing could be used to create "a genius mathematician, a brilliant musician or a soldier, a man who can fight without fear, compassion, regret or pain."
"[Such uses] would be considered using gene editing for 'enhancement,'" said Dr. Zubin Master, an associate professor of biomedical ethics at the Mayo Clinic, who noted that a series of studies have strongly suggested that members of the public, in the U.S. and around the world, are much less amenable to the prospect of gene editing for these purposes than for the treatment of illness and disease.
Putin's comments were made in 2017, before news of He's experiment broke; since then no country has moved to continue experiments on germline editing (although one Russian IVF specialist, Denis Rebrikov, appears ready to do so, if given approval). Master noted that the World Health Organization has an 18-person committee currently dedicated to considering these questions. The Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing first convened in March 2019; that July, it issued a recommendation to regulatory and ethics authorities in all countries to refrain from approving clinical application requests for work on human germline genome editing—the kind of alterations to genetic cells used by He. The committee's report and a fleshed-out set of guidelines is expected after its final meeting, in Geneva this September (unless the COVID-19 pandemic disrupts the timeline).
Regardless of the WHO's report, in the U.S., all regulations of new medical procedures are overseen at the federal level, subjected to extensive regulatory review by the FDA; the chance of any doctor or company going rogue is minimal to none. Likewise, the challenges we face are more on the regulatory end of the spectrum than the Gattaca end. Dr. Stephanie Malia Fullerton, a bioethics professor at the University of Washington, pointed out that eugenics not only typically involves state-sponsored control of reproduction, but requires a much more clearly delineated genetic basis of common complex traits—indeed, SCN9A is one way to get to pain, but is not the only source—and suggested that current concerns about over-prescribing opioids are a more pressing question for society to address.
In fact, Navega Therapeutics, based in San Diego, hopes to find out whether the intersection of this research into SCN9A and CRISPR would be an effective way to address the U.S. opioid crisis. Currently in a preclinical funding stage, Navega's approach focuses on editing epigenetic molecules attached to the basic DNA strand—the idea is that the gene's expression can be activated or suppressed rather than removed entirely, reducing the risk of unwanted side effects from permanently altering the genetic code.
As these studies focused on the sensation of pain go forward, what we are likely to see simultaneously is the use of CRISPR to target diseases that are the root causes of that pain. Last summer, Victoria Gray, a Mississippi woman with sickle cell disease was the second-ever person to be treated with CRISPR therapy in the U.S. The disease is caused by a genetic mutation that creates malformed blood cells, which can't carry oxygen as normal and get stuck inside blood vessels, causing debilitating pain. For the study, conducted in concert with CRISPR Therapeutics, of Cambridge, Mass., cells were removed from Gray's bone marrow, modified using CRISPR, and infused back into her body, a technique called ex vivo editing.
In early February this year, researchers at the University of Pennsylvania published a study on a first-in-human phase 1 clinical trial, in which three patients with advanced cancer received an infusion of ex vivo engineered T cells in an effort to improve antitumor immunity. The modified cells persisted for up to nine months, and the patients experienced no serious adverse side effects, suggesting that this sort of therapeutic gene editing can be performed safely and could potentially allow patients to avoid the excruciating process of chemotherapy.
Then, just this spring, researchers made another advance: The first attempt at in vivo CRISPR editing—where the edits happen inside the patient's body—is currently underway, as doctors attempt to treat a patient blinded by Leber congenital amaurosis, a rare genetic disorder. In an Oregon study sponsored by Editas Medicine and Allergan, the patient, a volunteer, was injected with a harmless virus carrying CRISPR gene-editing machinery; the hope is that the tool will be able to edit out the genetic defect and restore production of a crucial protein. Based on preliminary safety reports, the study has been cleared to continue, and data on higher doses may be available by the end of 2020. Editas Medicine and CRISPR Therapeutics are joined in this sphere by Intellia Therapeutics, which is seeking approval for a trial later this year on amyloidosis, a rare liver condition.
For any such treatment targeting SCN9A to make its way to human subjects, it would first need to undergo years' worth of testing—on mice, on primates, and then on volunteer patients after an extended informed-consent process. If everything went perfectly, Urnov estimates it could take at least three to four years end to end and cost between $5 and 10 million—but that "if" is huge.
"The idea of a regular human being, genetically pure of pain?"
And as that happens, "we need to have appropriate venues where we deliberate and consider the ethical, legal and social implications of gene editing as a society," Master said. CRISPR itself is open-source, but its application is subject to the approval of governments, institutions, and societies, which will need to figure out where to draw the line between miracle treatments and playing God. Something as unpleasant and ubiquitous as pain may in fact be the most appropriate place to start.
"The pain circuit is very old," Urnov said. "We have evolved with the senses that we have, and have become the species that we are, as a result of who we are, physiologically. Yes, I take Advil—but when I get a headache! The idea of a regular human being, genetically pure of pain?... The permanent disabling or turning down of the pain sensation, for anything other than a medical reason? … That seems to be challenging Mother Nature in the wrong ways."
Five Memorable Animals Who Expanded the Scientific Frontier
Laika, a gene-edited pig, was named in honor of the first living creature to orbit the earth, a stray dog named Laika.
Untold numbers of animals have contributed to science, in ways big and small. Studying cows and cowpox helped English doctor Edward Jenner create a smallpox vaccine; Ivan Pavlov's experiments on dogs' reactions to external stimuli heavily influenced modern behavioral psychology.
We have these five animals to thank for some of our most important scientific advancements, from space travel to better organ replacement options.
Scientists still work with rats, rabbits, and other mammals to test cosmetics and pharmaceuticals and to conduct infectious disease research. Most of these animals remain nameless and unknown to the public, but over the years, certain individuals have had an outsize effect. We have these five animals to thank for some of our most important scientific advancements, from space travel to better organ replacement options.
1) LAIKA THE DOG
Laika was the first living creature ever to orbit the Earth. In October 1957, the Soviet Sputnik I ship had made history as the first man-made object sent into Earth's orbit; Premier Nikita Khrushchev was keen to gain another Space Race victory by sending up a canine cosmonaut.
Laika ("barker" in Russian), was a stray dog, reportedly a husky-spitz mix, recruited among several other female strays for the trip. Although the scientists put extensive work into preparing Laika and the other canine finalists—evaluating their reactions to air-pressure variations, training them to adapt to pelvic sanitation devices meant to contain waste, and eventually having them live in pressurized capsules for weeks—there was no expectation that the dog would return to Earth, and only one meal's worth of food was sent up with her.
Sputnik II, six times heavier than its predecessor, launched on November 3, 1957. Soviet broadcasts reported that Laika, fitted out with surgically implanted devices to monitor her heart rate, blood pressure, and breathing rates, survived until November 12; the spacecraft stayed in orbit for five more months, burning up when it re-entered the atmosphere.
At the time, the Sputnik II team reassured the world that Laika had died painlessly of oxygen deprivation. It was only decades later, in the 1990s, that Oleg Gazenko—one of the scientists and dog trainers assigned to the mission—revealed that Laika had died 5 to 7 hours after launch from a combination of heat and stress. The capsule had overheated, probably as a result of the rushed preparation; after the fourth orbit, the temperature inside Sputnik was over 90 degrees, and it's doubtful she could have survived much past that. "The more time passes, the more I'm sorry about it. We shouldn't have done it," Gazenko said. "We did not learn enough from the mission to justify the death of the dog."
Yet even the four or five orbits that Laika did complete were enough to spur scientists to press on in the effort to send a human into space.
2) HAM THE CHIMP
Four years after Laika's ill-fated flight, a chimpanzee named Ham entered suborbital flight in the American Project Mercury MR-2 mission on January 31, 1961, becoming the first hominid in space—and unlike Laika, he returned to Earth, alive, after a 16-minute flight.
Even though Ham's flight was not destined for orbit, the spacecraft and booster used on his trip were the same combination intended for the first (human) American's trip later that year. If he came back unharmed, NASA's medical team would be prepared to okay astronaut Alan Shepard's flight.
For approximately 18 months before liftoff, Ham was trained to perform simple tasks, like pushing levers, in response to visual and auditory cues. (If he failed, he received an electric shock; correct performance earned him a treat. Pavlov would have been pleased.)
At 37 pounds, Ham was also the heaviest animal to ever make it to space. His vital signs and movements were monitored from Earth, and after a light electric shock from the ground team reminded him of his tasks, he performed his lever-pushing just a bit slower than he had on Earth, verifying that motion would not be seriously impaired in space.
Less than three months after Ham returned to Earth, on April 12, 1961, Soviet cosmonaut Yuri Gagarin became the first human to complete an orbital flight; Shepard was close behind, successfully crewing the MR-3 mission on May 5. For his part, Ham "retired" to the National Zoo in Washington D.C. for 17 years, before being transferred to the North Carolina Zoological Park; he died of liver failure in 1983 at age 26. His grave is at the International Space Hall of Fame in New Mexico.
3) KOKO THE GORILLA
A western lowland gorilla born at the San Francisco Zoo, Hanabi-ko, or "Koko," became famous in the 1970s for her cognitive and communicative abilities. Psychologist Francine "Penny" Patterson, then a doctoral student at Stanford, chose Koko to work on a language research project, teaching her American Sign Language; by age four, Koko demonstrated the ability both to make up new words and to combine known words to express herself creatively, as opposed to simply mimicking her trainer.
Koko's work with Patterson reflected levels of cognition that were higher than non-human primates had previously been thought to have; by the end of her life, her language skills were roughly equivalent to a young child's, with a vocabulary of around 1,000 signs and the ability to understand 2,000 words of spoken English.
An especially impactful study in 2012 showed that Koko had learned to play the recorder, revealing an ability for voluntary breath control that scientists had previously thought was linked closely to speech and could only be developed by humans. Barbara J. King, a biological anthropologist, suggested that Koko's immersion in a human environment may have helped her develop such a skill, and that it might be misleading to consider similar abilities "innate" or lacking in either humans or non-human primates.
Koko's displays of emotions also fascinated the public, especially those that seemed to closely mirror humans': she cared for pet kittens; appeared on Mr. Rogers' Neighborhood and untied the host's shoes for him; acted playfully with Robin Williams during a visit from him, and later expressed grief when told about the comedian's death. Koko died in her sleep in June 2018, at age 46. Patterson continues to run The Gorilla Foundation, which is dedicated to using inter-species communication to motivate conservation efforts.
4) DOLLY THE SHEEP
Dolly—named after country singer Dolly Parton—was the first mammal ever to be cloned from an adult somatic cell, using the process of nuclear transfer. She was born in 1996 as part of research by scientists Keith Campbell and Ian Wilmut of the University of Edinburgh.
By taking a donor cell from an adult sheep's mammary gland, using it to replace the cell nucleus of an unfertilized, developing egg cell, and then bringing the resultant embryo to term, Campbell and Wilmut proved that even a mature cell (one that had developed to perform mammary gland functions) could revert to an embryonic state and go on to develop into any and all parts of a mammal.
Although cloned livestock are legal in the U.S.—the FDA approved the practice in 2008, after determining that there was no difference between the meat and milk of cattle, pigs, and goats—Dolly has had an even bigger impact on stem cell research. The successful test of nuclear transfer proved that it was possible to change a cell's gene expression by changing its nucleus.
Japanese stem cell biologist Shinya Yamanaka, inspired by the birth of Dolly, won the Nobel Prize in 2012 for his adaptation of the technique. He developed induced pluripotent stem cells (iPS cells) by chemically reverting mature cells back to an embryonic-like blank state that is highly desirable for disease research and treatment. This technique allows researchers to work with such stem cells without the ethically charged complication of having to destroy a human embryo in the process.
5) LAIKA THE PIG
Named in honor of the dog who made it to space, the second science-famous Laika was a genetically engineered pig born in China in 2015 as a result of gene editing carried out by Cambridge, MA startup eGenesis and collaborators.* eGenesis aims to create pigs whose organs—hearts, kidneys, lungs, and more—are safe to transplant into people.
Using animal organs in humans (xenotransplantation) is tricky: the immune system is very good at recognizing interlopers, and the human body can start to reject an organ from another species in as little as five minutes. But pigs are otherwise exceptionally good potential donors for humans: their organs' sizes and functions are very similar, and their quick gestation and maturation make them attractive from an efficiency standpoint, given that twenty Americans die every day waiting for organ donors.
Perhaps unsurprisingly, Dolly the sheep helped move xenotransplantation forward. In the 1990s, immunologist David Sachs was able to use a similar cloning method to eliminate alpha-gal, an enzyme that is produced by most animals with immune systems, including pigs—but not humans. Since our immune systems don't recognize alpha-gal, attacks on that enzyme are a major cause of organ rejection. Sachs' experiments increased the survival time of pig organs in primates to weeks: a huge improvement, but not nearly enough for someone in need of a liver or heart.
The advent of CRISPR technology, and the ability to edit genes, has allowed another leap. In 2015, researchers at eGenesis used targeted gene-editing to eliminate the genes for porcine endogenous retroviruses from pig kidney cells. These viral elements are part of all pigs' genomes and pose a potentially high risk of infecting human cells. (After the HIV/AIDS crisis especially, there was a lot of anxiety about potentially introducing a new virus into the human population.)
The eGenesis lab used nuclear transfer to embed the edited nuclei into egg cells taken from a normal pig; and Laika was born months later—without the dangerous viral genes. eGenesis is now working to make the organs even more humanlike, with the goal of one day providing organs to every human patient in need.
*[Disclosure: In 2019, eGenesis received a series B investment from Leaps By Bayer, the funding sponsor of leapsmag. However, leapsmag is editorially independent of Bayer and is under no obligation to cover companies they invest in.]
[Correction, March 3, 2020: Laika the gene-edited pig was born in China, not Cambridge, and eGenesis is pursuing xenotransplant programs that include heart, kidney, and lung, but not skin, as originally written.]
Three Big Biotech Ideas to Watch in 2020—And Beyond
Body-on-a-chip, prime editing, and gut microbes all are poised to make a big impact in 2020.
1. Happening Now: Body-on-a-Chip Technology Is Enabling Safer Drug Trials and Better Cancer Research
Researchers have increasingly used the technology known as "lab-on-a-chip" or "organ-on-a-chip" to test the effects of pharmaceuticals, toxins, and chemicals on humans. Rather than testing on animals, which raises ethical concerns and can sometimes be inaccurate, and human-based clinical trials, which can be expensive and difficult to iterate, scientists turn to tiny, micro-engineered chips—about the size of a thumb drive.
It's possible that doctors could one day take individual cell samples and create personalized treatments, testing out any medications on the chip.
The chips are lined with living samples of human cells, which mimic the physiology and mechanical forces experienced by cells inside the human body, down to blood flow and breathing motions; the functions of organs ranging from kidneys and lungs to skin, eyes, and the blood-brain barrier.
A more recent—and potentially even more useful—development takes organ-on-a-chip technology to the next level by integrating several chips into a "body-on-a-chip." Since human organs don't work in isolation, seeing how they all react—and interact—once a foreign element has been introduced can be crucial to understanding how a certain treatment will or won't perform. Dr. Shyni Varghese, a MEDx investigator at the Duke University School of Medicine, is one of the researchers working with these systems in order to gain a more nuanced understanding of how multiple different organs react to the same stimuli.
Her lab is working on "tumor-on-a-chip" models, which can not only show the progression and treatment of cancer, but also model how other organs would react to immunotherapy and other drugs. "The effect of drugs on different organs can be tested to identify potential side effects," Varghese says. In addition, these models can help the researchers figure out how cancers grow and spread, as well as how to effectively encourage immune cells to move in and attack a tumor.
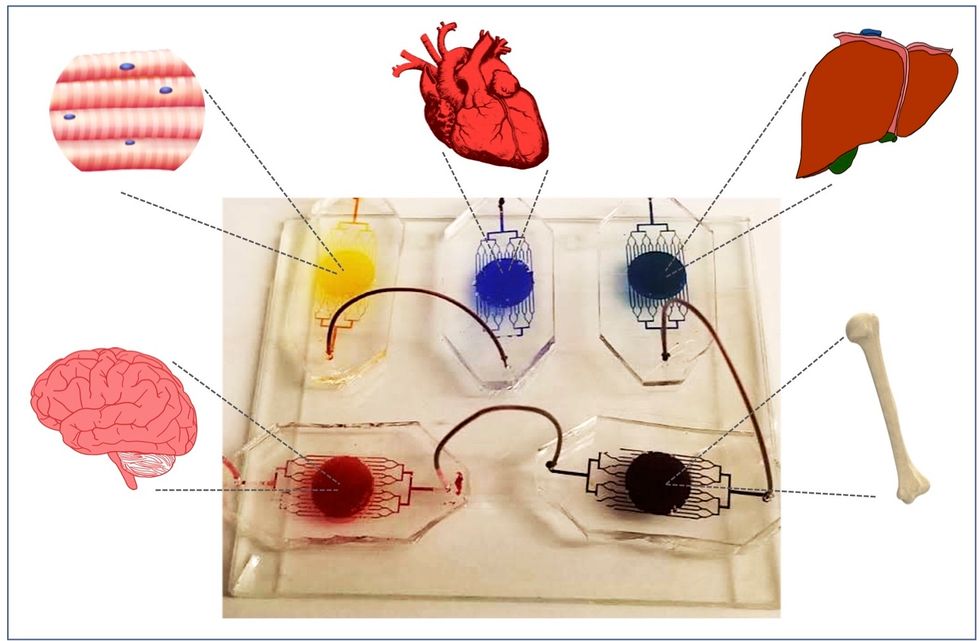
One body-on-a-chip used by Dr. Varghese's lab tracks the interactions of five organs—brain, heart, liver, muscle, and bone.
As their research progresses, Varghese and her team are looking for ways to maintain the long-term function of the engineered organs. In addition, she notes that this kind of research is not just useful for generalized testing; "organ-on-chip technologies allow patient-specific analyses, which can be used towards a fundamental understanding of disease progression," Varghese says. It's possible that doctors could one day take individual cell samples and create personalized treatments, testing out any medications on the chip for safety, efficacy, and potential side effects before writing a prescription.
2. Happening Soon: Prime Editing Will Have the Power to "Find and Replace" Disease-Causing Genes
Biochemist David Liu made industry-wide news last fall when he and his lab at MIT's Broad Institute, led by Andrew Anzalone, published a paper on prime editing: a new, more focused technology for editing genes. Prime editing is a descendant of the CRISPR-Cas9 system that researchers have been working with for years, and a cousin to Liu's previous innovation—base editing, which can make a limited number of changes to a single DNA letter at a time.
By contrast, prime editing has the potential to make much larger insertions and deletions; it also doesn't require the tweaked cells to divide in order to write the changes into the DNA, which could make it especially suitable for central nervous system diseases, like Parkinson's.
Crucially, the prime editing technique has a much higher efficiency rate than the older CRISPR system, and a much lower incidence of accidental insertions or deletions, which can make dangerous changes for a patient.
It also has a very broad potential range: according to Liu, 89% of the pathogenic mutations that have been collected in ClinVar (a public archive of human variations) could, in principle, be treated with prime editing—although he is careful to note that correcting a single genetic mutation may not be sufficient to fully treat a genetic disease.
Figuring out just how prime editing can be used most effectively and safely will be a long process, but it's already underway. The same day that Liu and his team posted their paper, they also made the basic prime editing constructs available for researchers around the world through Addgene, a plasmid repository, so that others in the scientific community can test out the technique for themselves. It might be years before human patients will see the results, and in the meantime, significant bioethical questions remain about the limits and sociological effects of such a powerful gene-editing tool. But in the long fight against genetic diseases, it's a huge step forward.
3. Happening When We Fund It: Focusing on Microbiome Health Could Help Us Tackle Social Inequality—And Vice Versa
The past decade has seen a growing awareness of the major role that the microbiome, the microbes present in our digestive tract, play in human health. Having a less-healthy microbiome is correlated with health risks like diabetes and depression, and interventions that target gut health, ranging from kombucha to fecal transplants, have cropped up with increasing frequency.
New research from the University of Maine's Dr. Suzanne Ishaq takes an even broader view, arguing that low-income and disadvantaged populations are less likely to have healthy, diverse gut bacteria, and that increasing access to beneficial microorganisms is an important juncture of social justice and public health.
"Basically, allowing people to lead healthy lives allows them to access and recruit microbes."
"Typically, having a more diverse bacterial community is associated with health, and having fewer different species is associated with illness and may leave you open to infection from bacteria that are good at exploiting opportunities," Ishaq says.
Having a healthy biome doesn't mean meeting one fixed ratio of gut bacteria, since different combinations of microbes can generate roughly similar results when they work in concert. Generally, "good" microbes are the ones that break down fiber and create the byproducts that we use for energy, or ones like lactic acid bacteria that work to make microbials and keep other bacteria in check. The microbial universe in your gut is chaotic, Ishaq says. "Microbes in your gut interact with each other, with you, with your food, or maybe they don't interact at all and pass right through you." Overall, it's tricky to name specific microbial communities that will make or break someone's health.
There are important corollaries between environment and biome health, though, which Ishaq points out: Living in urban environments reduces microbial exposure, and losing the microorganisms that humans typically source from soil and plants can reduce our adaptive immunity and ability to fight off conditions like allergies and asthma. Access to green space within cities can counteract those effects, but in the U.S. that access varies along income, education, and racial lines. Likewise, lower-income communities are more likely to live in food deserts or areas where the cheapest, most convenient food options are monotonous and low in fiber, further reducing microbial diversity.
Ishaq also suggests other areas that would benefit from further study, like the correlation between paid family leave, breastfeeding, and gut microbiota. There are technical and ethical challenges to direct experimentation with human populations—but that's not what Ishaq sees as the main impediment to future research.
"The biggest roadblock is money, and the solution is also money," she says. "Basically, allowing people to lead healthy lives allows them to access and recruit microbes."
That means investment in things we already understand to improve public health, like better education and healthcare, green space, and nutritious food. It also means funding ambitious, interdisciplinary research that will investigate the connections between urban infrastructure, housing policy, social equity, and the millions of microbes keeping us company day in and day out.







